
.png)


























This fragment is available on downloads.html
This publication includes IP covered under the following statements.
| Type | Reference | Content |
|---|---|---|
| web | github.com | Consolidated CDA (C-CDA), published by Health Level Seven. This guide is not an authorized publication; it is the continuous build for version 5.0.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/CDA-ccda/ and changes regularly. See the Directory of published versions |
| web | www.iso.org |
ISO maintains the copyright on the country codes, and controls its use carefully. For further details see the ISO 3166 web page: https://www.iso.org/iso-3166-country-codes.html
Show Usage
|
| web | www.nubc.org |
Licensing information can be found here
These codes are listed within the UB-04 Data Specifications Manual. The Official UB-04 Data Specifications Manual, copyrighted by the American Hospital Association, is the only official source of UB-04 billing information adopted by the National Uniform Billing Committee. No other publication—governmental or private/commercial—can be considered authoritative. The AHA wants to make you aware that the use of codes, descriptions, or any other content contained in the manual to be used in a software application, publication, or any other derivative work must be properly licensed by the AHA. If your organization uses or intends to use any of the codes or other related content from the manual in this manner, please contact the AHA’s licensing manager, Tim Carlson, at 312.893.6816 or tcarlson@aha.org
Show Usage
|
| web | ucum.org |
The UCUM codes, UCUM table (regardless of format), and UCUM Specification are copyright 1999-2009, Regenstrief Institute, Inc. and the Unified Codes for Units of Measures (UCUM) Organization. All rights reserved. https://ucum.org/trac/wiki/TermsOfUse
Show Usage
|
| web | github.com | HL7 has created a style sheet available for the community to use, as is, or to customize for their vendor’s implementations. The HL7 CDA style sheet is housed in the CDA-core-xsl GitHub repository. |
| web | github.com | CDA defines a standard schema, based on the HL7 RIM, for all CDA documents. The XML Schema is designed to achieve the intentions of the CDA architecture. Please see section The "A" in CDA for more information. When there is a need to represent information where there is no suitable representation in the schema, the CDA standard permits extensions to be developed. The HL7 Structured Documents Work Group (SDWG) maintains a complete list of CDA R2 extensions that are approved for use within the sdtc namespace. These extensions exist in the stdc schema. The most current CDA Schema is housed in the HL7 CDA GitHub repository. |
| web | carequality.org | This Supporting Guidance Page provides guidance aimed at increasing consistency in the way data in C-CDA templates are represented and used across all implementations. The information was derived from learnings gleaned since 2012 from cross vendor C-CDA implementations, C-CDA Implementation-A-Thons and quality review of HIE C-CDAs such as CareEquality . |
| web | hl7-c-cda-examples.herokuapp.com | Prior Address |
| web | github.com | Because of the variability of how care team members are represented in the header, and because there is a lack of normative guidance on which header items must be rendered, it is recommended that receiving systems should render ALL the participants in the header, rather than only rendering participants of a particular type. The HL7 CDA style sheet supports complete participant rendering. |
| web | www.commonwellalliance.org | For more information and Guidance see Concise Consolidated CDA: Deploying Encounter Summary CDA Documents with Clinical Notes . |
| web | www.jointcommission.org | The Discharge Summary is a document which summarizes a patient's admission to a hospital, LTPAC provider, or other setting. It provides information for the continuation of care following discharge. The Joint Commission requires the following information to be included in the Discharge Summary: |
| web | www.tabers.com | The Progress Note represents a patient’s clinical status during a hospitalization, outpatient visit, treatment with a LTPAC provider, or other healthcare encounter. Taber’s medical dictionary defines a Progress Note as “An ongoing record of a patient's illness and treatment. Physicians, nurses, consultants, and therapists record their notes concerning the progress or lack of progress made by the patient between the time of the previous note and the most recent note. A Progress Note is not a re-evaluation note. A Progress Note is not intended to be a Progress Report for Medicare. Medicare B Section 1833(e) defines the requirements of a Medicare Progress Report. The Joint Document Content Work Group recommends use of the Progress Note document template to represent an encounter summary for a non-inpatient setting in Chapter 2.2 Outpatient/Ambulatory Summary (Progress Note Document). The Progress Note document template does not include any required sections and the open nature of the template enables Content Creators to include the right sections to express the source data or the needed sections to satisfy the requirements of Content Consumers. |
| web | www.commonwellalliance.org | The Progress Note represents a patient’s clinical status during a hospitalization, outpatient visit, treatment with a LTPAC provider, or other healthcare encounter. Taber’s medical dictionary defines a Progress Note as “An ongoing record of a patient's illness and treatment. Physicians, nurses, consultants, and therapists record their notes concerning the progress or lack of progress made by the patient between the time of the previous note and the most recent note. A Progress Note is not a re-evaluation note. A Progress Note is not intended to be a Progress Report for Medicare. Medicare B Section 1833(e) defines the requirements of a Medicare Progress Report. The Joint Document Content Work Group recommends use of the Progress Note document template to represent an encounter summary for a non-inpatient setting in Chapter 2.2 Outpatient/Ambulatory Summary (Progress Note Document). The Progress Note document template does not include any required sections and the open nature of the template enables Content Creators to include the right sections to express the source data or the needed sections to satisfy the requirements of Content Consumers. |
| web | journals.lww.com | The idea of improving the communication of information contained in clinical notes has been around for decades. Long before C-CDA, a documentation methodology called “SOAP (Subjective, Objective, Assessment, and Plan) notes” was invented. The birth of the problem-oriented medical record (POMR) and SOAP note marked an epoch in the history of health care. Dr. Lawrence Weed, developer of the SOAP note and professor of medicine and pharmacology at Yale University , challenged conventional medical documentation and advocated for a scientific structure to frame clinical reasoning in the 1950s. Today, the SOAP note is the most common method of documentation used by providers to input notes into patients’ medical records. They allow providers to record and share information in a universal, systematic and easy to read format. Ineffective communication contributes to the top causes of sentinel events and continues to be an unremitting area for refinement. |
| web | www.hcinnovationgroup.com | While no longer active today, the HIMSS Health Story Project provided education to the health IT community on tools and resources to aid in the creation of comprehensive electronic records that tell a patient's complete health story. Some especially educational concepts from this initiative are summarized in the following chapters. |
| web | www.ihe.net | Sharing documents requires different views based on user needs. HL7 Relevant and Pertinent Survey highlights the need to enhance CDA document rendering. CDA uses XML stylesheets with @styleCode, as defined by IHE's MCV Profile. Refer to IHE Multiple Content Views (MCV) Profile for details and examples. |
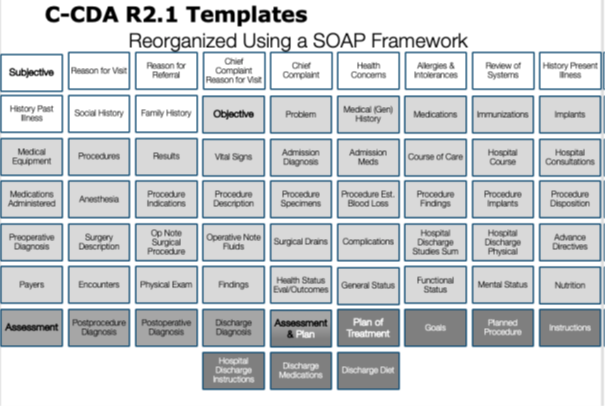
| web | www.himss.org | As explained in the Health Story Roundtable presentation titled The Storytelling Power of C-CDA , understanding the purpose of the C-CDA section templates is not facilitated by considering them in alphabetical order. The C-CDA Implementation Guide presents them in alphabetical order to speed access for readers. Considering the C-CDA section templates using the SOAP framework makes it easier to see how these sections can be used in a structured document to express the content of a clinical SOAP note. |
| web | www.himss.org | A study by the Association for Healthcare Documentation Integrity, presented at the HIMSS Health Story Project Roundtable on March 5, 2019 , showed that consistent style in healthcare records enhances clear communication, boosts patient safety, and improves information exchange. Applying style standards to human-readable text can reduce physician burden, with a focus on improving the representation of temporal date/time information in the narrative text. While temporal details are vital for clinical understanding, excessive date/time precision can overwhelm or mislead. The Roundtable urged C-CDA creators to ensure that date/time information in the Narrative Block is relevant, pertinent, and appropriately styled to ease physician burden. |
| web | hl7-c-cda-examples.herokuapp.com | Visit HL7 CDA Example Search |
| web | hl7-c-cda-examples.herokuapp.com | How to represent Outpatient Encounter with Diagnoses |
| web | hl7-c-cda-examples.herokuapp.com | How to reference Hospital Discharge Encounter with Billable Diagnoses |
| web | hl7-c-cda-examples.herokuapp.com |
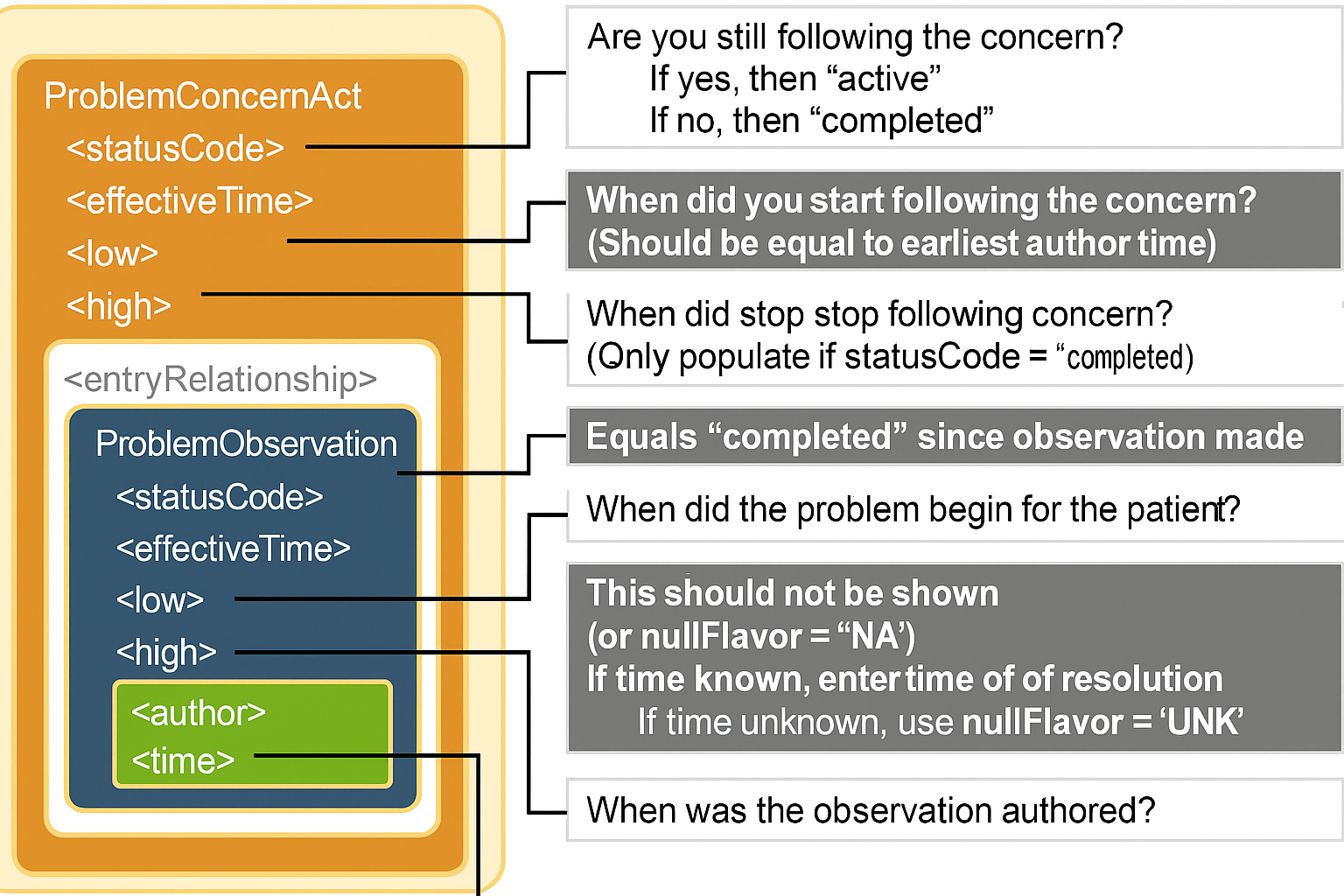
A Problem Concern Act can contain one or more Problem Observations (templateId 2.16.840.1.113883.10.20.22.4.4). In practice, most EHRs do not support this template design. See best practice guidance below for implementers who do not support problem concern tracking at this time. Visit HL7 CDA Example Search for representing the expression “No Known Problems” |
| web | hl7-c-cda-examples.herokuapp.com |
A Problem Concern Act can contain one or more Problem Observations (templateId 2.16.840.1.113883.10.20.22.4.4). In practice, most EHRs do not support this template design. See best practice guidance below for implementers who do not support problem concern tracking at this time. Visit HL7 CDA Example Search for representing the expression “No Known Problems” |
| web | hl7-c-cda-examples.herokuapp.com | Visit HL7 CDA Example Search |
| web | hl7-c-cda-examples.herokuapp.com | C-CDA Examples Task Force Problem Section entry examples |
| web | hl7-c-cda-examples.herokuapp.com |
The optional Problem Status Observation template (identifier: urn:oid:2.16.840.1.113883.10.20.22.4.6) represents a clinical judgement of the status of the problem. negationInd of "true" coupled with an observation/value of SNOMED code 55607006 "Problem" indicates that the patient has no known conditions . |
| web | hl7-c-cda-examples.herokuapp.com |
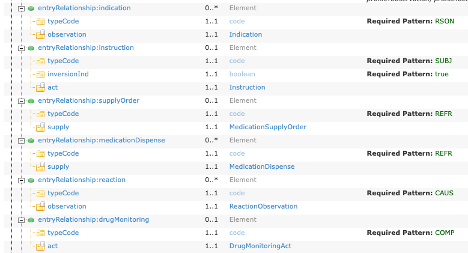
An Allergy Concern Act can contain one or more Allergy - Intolerance Observations (templateId .16.840.1.113883.10.20.22.4.7). In practice, most EHRs do not support this template design. See best practice guidance below for implementers who do not support allergy concern tracking at this time. Visit HL7 CDA Example Search for representing the expression “No Known Allergies”, “No Known Medication Allergies” . |
| web | hl7-c-cda-examples.herokuapp.com |
An Allergy Concern Act can contain one or more Allergy - Intolerance Observations (templateId .16.840.1.113883.10.20.22.4.7). In practice, most EHRs do not support this template design. See best practice guidance below for implementers who do not support allergy concern tracking at this time. Visit HL7 CDA Example Search for representing the expression “No Known Allergies”, “No Known Medication Allergies” . |
| web | hl7-c-cda-examples.herokuapp.com | Visit HL7 CDA Example Search for additional examples showing allergies to specific medication. |
| web | hl7-c-cda-examples.herokuapp.com | Example 40: Allergy concern for food allergy to eggs Please see the Example Task Forces Allergy Examples |
| web | hl7-c-cda-examples.herokuapp.com |
The optional Allergy Status Observation template (identifier: urn:oid: 2.16.840.1.113883.10.20.22.4.28) signifies the status of the allergy-intolerance. Within the Allergy-Intolerance Observation, there is a Reaction Observation detailing the associated reaction, including its severity. Additionally, a Criticality Observation is included to indicate the seriousness of the allergy-intolerance, with possible values being high, low, or unable to assess criticality. Visit HL7 CDA Example Search for representing the expression “No Known Allergies. A negationInd of "true" coupled with an observation/value of SNOMED code 41919907. "Allergy to substance (disorder)" indicates that the patient has no known allergies. |
| web | hl7-c-cda-examples.herokuapp.com | Visit HL7 CDA Example Search for additional examples for allergies |
| web | hl7-c-cda-examples.herokuapp.com | Example 46: No Implanted Devices |
| web | hl7-c-cda-examples.herokuapp.com | Table 52: Goal Observation Template Example task Force Goal examples Example 48: Goal Observation narrative |
| web | www.betterhealth.vic.gov.au | Pathology is the superset domain that encompasses several subdisciplines: anatomic pathology, chemical pathology, clinical pathology, forensic pathology, genetic pathology, hematology, immunopathology, etc.( Pathology Tests Definition ). Therefore, a laboratory test is a type of pathology test. |
| web | hl7-c-cda-examples.herokuapp.com | C-CDA Example Task Force Result Section examples |
| web | hl7-c-cda-examples.herokuapp.com | Table 60: Vital Signs Organizer Template C-CDA Examples Task Force Vital Sign Section examples Example 57: Vital Signs Organizer |
| web | docs.google.com | When representing medications, consideration needs to be given to the way date/time intervals are represented. See Chapter 5.1.10.2 Date/Time Precision for additional information about how to represent and interpret date ranges that use an effectiveTime/low and effectiveTime/high. The CDA Example Task Force includes a document that summarizes commonly used medication frequencies . |
| web | hl7-c-cda-examples.herokuapp.com | C-CDA Examples Task Force Medication Examples |
| web | hl7-c-cda-examples.herokuapp.com | C-CDA Examples Task Force Immunization Examples |
| web | hl7-c-cda-examples.herokuapp.com | How to represent Procedure Activity Procedure |
| web | hl7-c-cda-examples.herokuapp.com | WE CARE |
| web | hl7-c-cda-examples.herokuapp.com | Glasgow Coma |
| web | snomed.info | SNOMED |
| web | unitsofmeasure.org | UCUM |
| web | www.nubc.org | AHA_NUBC_PatientDischargeStatus |
| web | www.ada.org | CDT |
| web | www.ama-assn.org | CPT |
| web | www.iso.org | ISO3166Part1 |
| web | nucc.org | NUCC_Provider_Taxonomy |
| web | nahdo.org | SOP |
| web | www.usps.com | USPSStateCodes |
| web | github.com | For those who prefer Schematron validation for C-CDA documents, HL7 created an open-source tool which generates Schematron and other validation files for implementation guides created with FHIR StructureDefinitions. While we plan to integrate this functionality into the IG Publisher in the future, you can currently find C-CDA Schematron in the implementation guide's GitHub repository . |
| web | github.com | For those who prefer Schematron validation for C-CDA documents, HL7 created an open-source tool which generates Schematron and other validation files for implementation guides created with FHIR StructureDefinitions. While we plan to integrate this functionality into the IG Publisher in the future, you can currently find C-CDA Schematron in the implementation guide's GitHub repository . |
Figure 5 Visualizing C-CDA section templates by applying the SOAP framework.png 
|
Figure 7 Logical design for Care Team Organizer Template.png 
|
Figure 8 Example of SDOH Grouping Value set in VSAC (Conditions).png .png)
|
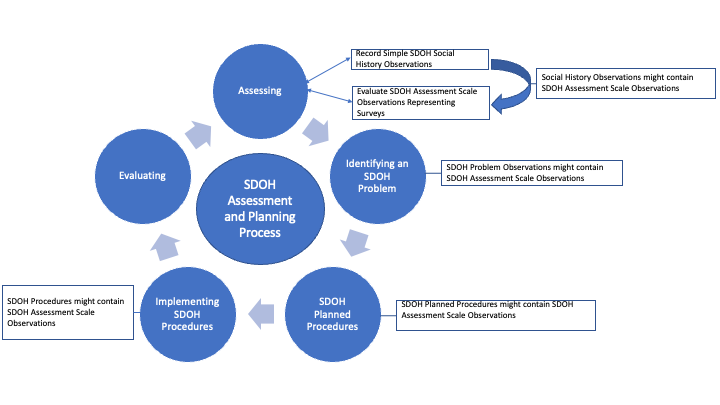
Figure 9 SDOH Assessment and Planning Process.png 
|
USCDI.png 
|
allergies-and-intolerances.png 
|
care-plan.png 
|
care-team-members.png 
|
cda_problemactandproblemobs_timing.png 
|
clinical-notes.png 
|
clinical-tests.png 
|
diagnostic-imaging.png 
|
encounter.png 
|
facility-information.png 
|
family-health-history.png 
|
goals-and-preferences.png 
|
health-insurance-information.png 
|
health-status-assessments.png 
|
immunizations.png 
|
laboratory.png 
|
medical-devices.png 
|
medications.png 
|
observation.png 
|
orders.png 
|
patient-demographics.png 
|
patient-summary-and-plan.png 
|
problems.png 
|
procedures.png 
|
profile_differential_element_types.png 
|
propose_a_change.png 
|
provenance.png 
|
resource_profile_info_tabs.png 
|
sample_profile_detailed_desc.png 
|
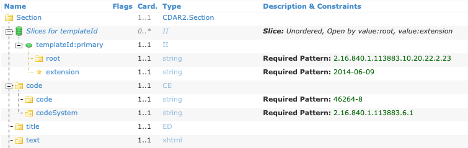
sample_profile_differential.png 
|
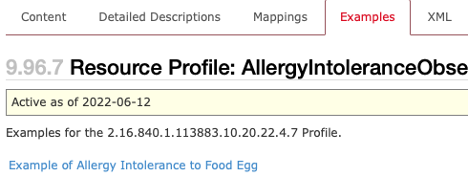
sample_profile_example.png 
|
sample_profile_structures.png 
|
sample_profile_usage.png 
|
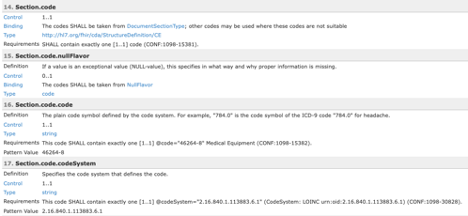
sample_profile_vocabulary.png 
|
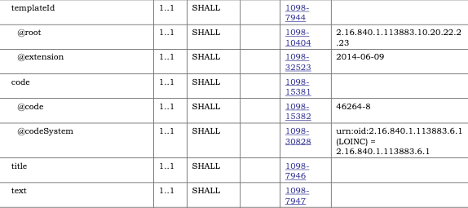
sample_template_constraint.png 
|
sample_template_constraint_doc.png 
|
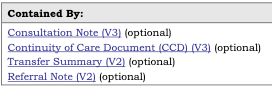
sample_template_contained_by.png 
|
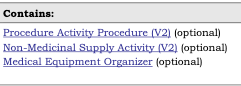
sample_template_contains.png 
|
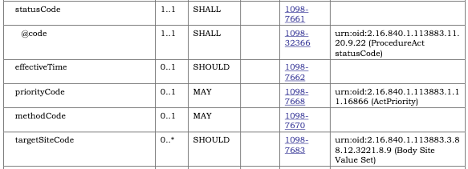
sample_template_vocabulary.png 
|
template_identifier.png 
|
templated-cda.png 
|
tree-filter.png 
|
vital-signs.png 
|