Consolidated CDA (C-CDA), published by Health Level Seven. This guide is not an authorized publication; it is the continuous build for version 5.0.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/CDA-ccda/ and changes regularly. See the Directory of published versions
This Supporting Guidance Page provides guidance aimed at increasing consistency in the way data in C-CDA templates are represented and used across all implementations. The information was derived from learnings gleaned since 2012 from cross vendor C-CDA implementations, C-CDA Implementation-A-Thons and quality review of HIE C-CDAs such as CareEquality.
This pages provides generalized document, section and entry guidance and additional guidance for specific types of C-CDA documents.
All of the guidance blocked in blue below represent non-mandatory recommendations, labeled as Best Practices. They are based on consensus insights and lessons learned that aim to improve interoperability, data quality, and implementation outcomes. While not not required for conformance, they are strongly encouraged. Instead of using conformance verbs like "SHALL", Best Practices use phases such as "is encouraged to" or "is strongly encouraged to" to reflect the recommended level of adherence.
C-CDA establishes two document header templates. These header templates establish consistent rules for populating the data elements in a C-CDA document header. Document header elements create context for the information contained in the body of the document.
The US Realm General Header is used for documents that are authored by clinicians and systems supporting clinicians, and the US Realm Patient Generated Header is used for documents that are authored by patients and systems supporting patients.
Each document template defined in C-CDA indicates the header template that the document is expected to conform to, and each also includes additional header constraints that are relevant to the type of clinical note being represented in the document. |
Additionally, document templates define the sections of content that SHALL, SHOULD, or MAY be present for exchanging clinical notes. Each section is identified with a code that tells the receiving systems the type of information in the section.
Further, the sections define entry templates which SHALL, SHOULD, or MAY be used to represent the information in each section using discrete representations that aid further computer processing of the clinical information in the section.
CDA context is set in the CDA header and applies to the entire document. Context can be overridden at the level of the section, and/or CDA entry. A document, in a sense, is a contextual wrapper for its contents. Assertions in the document header are typically applicable to statements made in the body of the document, unless overridden. For instance, the patient identified in the header is assumed to be the subject of observations described in the body of the document, unless a different subject is explicitly stated. The author identified in the header is assumed to be the author of the information in the sections and entries of the document, unless a different author is explicitly identified on a section or on individual entries. The objective of the CDA context rule is to make these practices explicit with relationship to the RIM, such that a computer will understand the context of a portion of a document the same way that a human interprets it.
CDA's approach to context, and the propagation of that context to nested document components, follows these design principles:
Context components that can be overridden at the level of a document section include: o Author o Confidentiality o Human language o Informant o Subject
Example 4: Section with narrative text and a nullFlavor for the author
This example explains that the author of the section is not known. Thus the author in the header does not conduct to be the author of the unparsed narrative information contained in the section.
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1"/>
<code code="10154-3" displayName="Chief complaint Reported"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Chief Complaint Section</title>
<text><content>No complaints, annual wellness exam</content>
<content>Author of this section not known.</content>
</text>
<author nullFlavor="UNK">
<time/>
<assignedAuthor>
<id/>
</assignedAuthor>
</author>
</section>
Reference: For additional information on Context Conduction mechanisms in CDA documents,see CDA Context in the HL7 CDA R2.0 Structure Definition Publication.
Reference: The HL7 Data Security for Privacy and HL7 Privacy Consent Directives implementation guides define how to override confidentiality context at the entry level.
All CDA documents include a structured header regardless if the document is a CDA document with a structured structuredBody element (a “structured document”) or a CDA document with a nonXMLBody element (an “unstructured document”). The structured header permits computer processing (parsing) to occur on its content.
The recordTarget represents the medical record that this document belongs to. In the uncommon case where a clinical document (such as a group encounter note) is placed into more than one patient chart, more than one recordTarget participants can be stated. The recordTarget records the administrative and demographic data of the patient whose health information is described by the clinical document; each recordTarget must contain at least one patientRole element.
The recordTarget contains many elements that hold core data for interoperability. The table below summarizes data elements present in the recordTarget.
Patient matching continues to be one of the major challenges for interoperability due to the lack of a universal patient identifier, similar to a Social Security Number, but used for healthcare.
| Data Element | Data element xPath |
|---|---|
| First Name | /ClinicalDocument/recordTarget/patientRole/patient/name/given[1] |
| Last Name | /ClinicalDocument/recordTarget/patientRole/patient/name/family |
| Previous Name | /ClinicalDocument/recordTarget/patientRole/patient/name/family/@qualifier |
| Middle Name | /ClinicalDocument/recordTarget/patientRole/patient/name/given[2] |
| Suffix | /ClinicalDocument/recordTarget/patientRole/patient/name/suffix |
| Administrative Gender | /ClinicalDocument/recordTarget/patientRole/patient/administrativeGenderCode |
| Birth Sex | Reference: Birth Sex |
| Date of Birth | /ClinicalDocument/recordTarget/patientRole/patient/birthTime |
| Race | /ClinicalDocument/recordTarget/patientRole/patient/raceCode |
| Ethnicity | /ClinicalDocument/recordTarget/patientRole/patient/ethnicGroupCode |
| Preferred Language (requires preferenceInd="true") | /ClinicalDocument/recordTarget/patientRole/patient/languageCommunication /ClinicalDocument/recordTarget/patientRole/patient/languageCommunication/preferenceInd |
| Address | /ClinicalDocument/recordTarget/patientRole/addr |
| Phone Number | /ClinicalDocument/recordTarget/patientRole/telecom |
Table 10: Patient Data Elements
C-CDA Content Creators are encouraged to include identifiers that improve the accuracy of automated patient matching mechanisms. Note: Testability would require business decisions to be made about what type of identifiers improve automated matching. |
C-CDA Content Creators who choose to include a non-null patient address are encouraged to follow the guidance in the Project US@ Technical Specification |
Example 5: recordTarget with demographic data
<recordTarget>
<patientRole>
<!-- Here is a public id that has an external meaning based on a root OID that is
publically identifiable. -->
<!-- root="1.3.6.1.4.1.41179.2.4" is the assigningAuthorityName for
DirectTrust's Patient/Consumer addresses "DT.org PATIENT" -->
<id root="1.3.6.1.4.1.41179.2.4" extension="lisarnelson@direct.myphd.us"
assigningAuthorityName="DT.org PATIENT"/>
<!-- More ids may be used. -->
<!-- Here is the patient's MRN at RVHS -->
<id root="2.16.840.1.113883.1.111.12345" extension="12345-0828"
assigningAuthorityName="River Valley Health Services local patient Medical
Record Number" />
<addr>
<streetAddressLine>1 Happy Valley Road</streetAddressLine>
<city>Westerly</city>
<state>RI</state>
<postalCode>02891</postalCode>
<country nullFlavor="UNK"/>
</addr>
<telecom use="WP" value="tel:+1-4013482345"/>
<telecom use="HP" value="tel:+1-4016412345"/>
<telecom value="mailto:lisanelson@gmail.com"/>
<telecom value="mailto:lisarnelson@direct.myphd.us"/>
<patient>
<name use="L">
<family>Nelson</family>
<given qualifier="CL">Lisa</given>
</name>
<administrativeGenderCode code="F" displayName="Female"
codeSystem="2.16.840.1.113883.5.1" codeSystemName="HL7
AdministrativeGender"/>
<birthTime value="19620828"/>
<maritalStatusCode code="M" displayName="Married"
codeSystem="2.16.840.1.113883.5.2"
codeSystemName="HL7 MaritalStatus"/>
<raceCode code="2106-3" displayName="White"
codeSystem="2.16.840.1.113883.6.238"
codeSystemName="CDC Race and Ethnicity"/>
<ethnicGroupCode code="2186-5" displayName="Not Hispanic or Latino"
codeSystem="2.16.840.1.113883.6.238" codeSystemName="CDC Race and
Ethnicity"/>
<languageCommunication>
<templateId root="2.16.840.1.113883.3.88.11.32.2"/>
<languageCode code="eng"/>
<preferenceInd value="true"/>
</languageCommunication>
</patient>
<providerOrganization>
<!-- This is a public id where the root is registered to indicate the
National Provider ID -->
<id root="2.16.840.1.113883.4.6" extension="1417947383"
assigningAuthorityName="National Provider ID"/>
</providerOrganization>
</patientRole>
</recordTarget>
Example 6: recordTarget including the provider organization context for the clinical documentation
<recordTarget>
<patientRole>
<!-- Here is a public id that has an external meaning based on a root OID that is publicly identifiable. -->
<!-- root="1.3.6.1.4.1.41179.2.4" is the assigningAuthorityName for
DirectTrust's Patient/Consumer addresses "DT.org PATIENT" -->
<id root="1.3.6.1.4.1.41179.2.4" extension="lisarnelson@direct.myphd.us"
assigningAuthorityName="DT.org PATIENT"/>
<!-- More ids may be used. -->
<!-- Here is the patient's MRN at RVHS -->
<id root="2.16.840.1.113883.1.111.12345" extension="12345-0828"
assigningAuthorityName="River Valley Health Services local patient Medical Record Number" />
<addr>
<streetAddressLine>1 Happy Valley Road</streetAddressLine>
<city>Westerly</city>
<state>RI</state>
<postalCode>02891</postalCode>
<country nullFlavor="UNK"/>
</addr>
<telecom use="WP" value="tel:+1-4013482345"/>
<telecom use="HP" value="tel:+1-4016412345"/>
<telecom value="mailto:lisanelson@gmail.com"/>
<telecom value="mailto:lisarnelson@direct.myphd.us"/>
<patient>
<name use="L">
<family>Nelson</family>
<given qualifier="CL">Lisa</given>
</name>
<administrativeGenderCode code="F" displayName="Female"
codeSystem="2.16.840.1.113883.5.1" codeSystemName="HL7 AdministrativeGender"/>
<birthTime value="19620828"/>
<maritalStatusCode code="M" displayName="Married" codeSystem="2.16.840.1.113883.5.2"
codeSystemName="HL7 MaritalStatus"/>
<raceCode code="2106-3" displayName="White" codeSystem="2.16.840.1.113883.6.238"
codeSystemName="CDC Race and Ethnicity"/>
<ethnicGroupCode code="2186-5" displayName="Not Hispanic or Latino"
codeSystem="2.16.840.1.113883.6.238" codeSystemName="CDC Race and Ethnicity"/>
<languageCommunication>
<templateId root="2.16.840.1.113883.3.88.11.32.2"/>
<languageCode code="eng"/>
<preferenceInd value="true"/>
</languageCommunication>
</patient>
<providerOrganization>
<!-- This is a public id where the root is registered to indicate the National Provider ID -->
<id root="2.16.840.1.113883.4.6" extension="1417947383"
assigningAuthorityName="National Provider ID"/>
<!-- This is a public id where the root indicates this is a Provider Direct Address. -->
<!-- root="1.3.6.1.4.1.41179.2.1" is the assigningAuthorityName for
DirectTrust's Covered Entity addresses "DT.org CE" -->
<id root="1.3.6.1.4.1.41179.2.1" extension="rvhs@rvhs.direct.md" assigningAuthorityName="DT.org CE (Covered Entity)"/>
<!-- By including a root OID attribute for a healthcare organization, you can use this information to
indicate a patient's MRN id at that organization.-->
<id root="2.16.840.1.113883.1.111.12345"
assigningAuthorityName="River Valley Health Services local patient Medical Record Number" />
<name>River Valley Health Services</name>
<telecom use="WP" value="tel:+1-4015394321"/>
<telecom use="WP" value="mailto:rvhs@rvhs.direct.md"/>
<addr>
<streetAddressLine>823 Main Street</streetAddressLine>
<city>River Valley</city>
<state>RI</state>
<postalCode>028321</postalCode>
<country>US</country>
</addr>
</providerOrganization>
</patientRole>
</recordTarget>
The CDA Examples Search tool provides useful examples showing the Patient Demographic data elements represented in a C-CDA document. The examples below show how to include information about a patient’s prior name or prior address in a C-CDA document. The relevance and pertinence of including this type of information in an exchange document remains a business decision to be made by organizations engaged in sharing information.
CDA Header: Patient Demographic Information
Example 7: How to represent Patient demographic Information
<recordTarget>
<patientRole>
<!-- The @root OID below (which is fictional) would be specific to an institution's
record identifier system. -->
<id root="2.16.840.1.113883.3.6132" extension="345678912-0154"/>
<!-- HP is "primary home" from valueSet 2.16.840.1.113883.1.11.10637 -->
<addr use="HP">
<!-- You can have multiple [1..4] streetAddressLine elements. Single shown below -->
<streetAddressLine>1436 Jennyhill Ln.</streetAddressLine>
<city>Hollywood</city>
<!-- 5 or 9 digit zip codes from valueSet 2.16.840.1.113883.3.88.12.80.2-->
<!-- PostalCode is required if the country is US. If country is not specified, it's
assumed to be US. If country -->
<!-- is something other than US, the postalCode MAY be present but MAY be bound to
different vocabularies -->
<postalCode>90068</postalCode>
<!-- State is required if the country is US. If country is not specified, it's
assumed to be US. -->
<!-- If country is something other than US, the state MAY be present but MAY be bound
to different vocabularies -->
<!-- OR is "Oregon" from valueSet 2.16.840.1.113883.3.88.12.80.1 -->
<state>CA</state>
<!-- US is the two digit code for "United States" -->
<country>US</country>
</addr>
<!-- MC is "mobile contact" from HL7 AddressUse 2.16.840.1.113883.5.1119 -->
<telecom value="tel:+1(565)867-5309" use="MC"/>
<!-- Multiple telecoms are possible -->
<telecom value="mailto://adam@diameterhealth.com" use="WP"/>
<patient>
<name use="L">
<given>Adam</given>
<family>Everyman</family>
</name>
<!-- From CDA R2 on administrativeGender Code: This attribute does not include terms
related to clinical gender.
Gender is a complex physiological, genetic and sociological concept
that requires multiple observations in order to be
comprehensively described. The purpose of this attribute is to
provide a high level classification that can additionally
be used for the appropriate allocation of inpatient bed assignment.-->
<!-- Sex at birth and gender identity may be specified in social history -->
<administrativeGenderCode code="M" codeSystem="2.16.840.1.113883.5.1"
displayName="Male" codeSystemName="AdministrativeGender"/>
<birthTime value="19621022"/>
<maritalStatusCode code="M" displayName="Married" codeSystem="2.16.840.1.113883.5.2"
codeSystemName="MaritalStatus"/>
<religiousAffiliationCode code="1013" displayName="Christian (non-Catholic, non-
specific)" codeSystem="2.16.840.1.113883.5.1076" codeSystemName="HL7 Religious Affiliation"/>
<!-- CDC Race and Ethnicity code set contains the five minimum race and the two
minimum ethnicity categories defined by OMB Standards -->
<raceCode code="2106-3" displayName="White" codeSystem="2.16.840.1.113883.6.238"
codeSystemName="OMB Standards for Race and Ethnicity"/>
<ethnicGroupCode code="2186-5" displayName="Not Hispanic or Latino"
codeSystem="2.16.840.1.113883.6.238" codeSystemName="OMB Standards for Race and Ethnicity"/>
<languageCommunication>
<languageCode code="eng"/>
<!-- "eng" is ISO 639-2 alpha-3 code for "English" -->
<modeCode code="ESP" displayName="Expressed spoken"
codeSystem="2.16.840.1.113883.5.60" codeSystemName="LanguageAbilityMode"/>
<proficiencyLevelCode code="E" displayName="Excellent"
codeSystem="2.16.840.1.113883.5.61" codeSystemName="LanguageAbilityProficiency"/>
<preferenceInd value="true"/>
</languageCommunication>
<!-- Multiple languages are permitted. Only one should have a preferenceInd = true -->
<languageCommunication>
<languageCode code="ita"/>
<!-- "ita" is ISO 639-2 alpha-3 code for "Italian" -->
<modeCode code="ESP" displayName="Expressed spoken"
codeSystem="2.16.840.1.113883.5.60" codeSystemName="LanguageAbilityMode"/>
<proficiencyLevelCode code="G" displayName="Good"
codeSystem="2.16.840.1.113883.5.61" codeSystemName="LanguageAbilityProficiency"/>
<!-- Patient's preferred language -->
<preferenceInd value="false"/>
</languageCommunication>
</patient>
</patientRole>
</recordTarget>
Example 8: How to represent Patient with Previous Name
<recordTarget>
<!-- Note: several other data elements (telecom, address, etc.) are required to meet C-CDA requirements -->
<patientRole>
<id extension="444222222" root="2.16.840.1.113883.4.1"/>
<patient>
<!-- SDWG reviewed and approved approach for recording a birth name and legal name -->
<name use="L">
<given>Alice</given>
<given>Jones</given>
<family>Newman</family>
</name>
<name>
<given qualifier="BR">Alicia</given> <!-- BR = Birth name-->
<given>Jones</given>
<family qualifier="BR">Newman</family> <!-- BR = Birth name-->
<!-- Optional valid time - best practice -->
<validTime>
<low value="20010505"/>
<high value="20010510"/>
</validTime>
</name>
</patient>
</patientRole>
</recordTarget>
Example 9: How to represent Patient with Prior Address
<!-- recordTarget example from C-CDA-Examples/Header/Patient Demographic Information
added multiple home addresses
-->
<recordTarget>
<patientRole>
<id root="2.16.840.1.113883.3.6132" extension="345678912-0154"/>
<!-- HP is "primary home" from valueSet 2.16.840.1.113883.1.11.10637
and in this instance represents patient's current address -->
<addr use="HP">
<streetAddressLine>152 Creek Lane</streetAddressLine>
<city>Shelburne</city>
<state>VT</state>
<postalCode>05455</postalCode>
<country>US</country>
<useablePeriod xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"
xsi:type="IVL_TS">
<low value="20110822"/>
</useablePeriod>
<!-- It is permissible to omit a low element to indicate current residence -->
<!-- It is permissible to omit a high element or use nullFlavor='NA' -->
</addr>
<addr use="H">
<streetAddressLine>191 S OAK AVE</streetAddressLine>
<city>BURLINGTON</city>
<state>VT</state>
<postalCode>05422</postalCode>
<country>US</country>
<useablePeriod xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"
xsi:type="IVL_TS">
<low value="20110131"/>
<high value="20110821"/>
</useablePeriod>
</addr>
<addr use="H">
<streetAddressLine>1141 W MAIN AVE</streetAddressLine>
<city>CHICAGO</city>
<state>IL</state>
<postalCode>60613</postalCode>
<country>US</country>
<useablePeriod xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"
xsi:type="IVL_TS">
<low value="20070513"/>
<high value="20110130"/>
</useablePeriod>
</addr>
<telecom value="tel:+1(565)867-5309" use="MC"/>
<telecom value="mailto://adam@diameterhealth.com" use="WP"/>
<patient>
<name use="L">
<given>Adam</given>
<family>Everyman</family>
</name>
<administrativeGenderCode code="M" codeSystem="2.16.840.1.113883.5.1"
displayName="Male" codeSystemName="AdministrativeGender"/>
<birthTime value="19621022"/>
<maritalStatusCode code="M" displayName="Married" codeSystem="2.16.840.1.113883.5.2"
codeSystemName="MaritalStatus"/>
<religiousAffiliationCode code="1013" displayName="Christian (non-Catholic, non-
specific)" codeSystem="2.16.840.1.113883.5.1076" codeSystemName="HL7 Religious Affiliation"/>
<raceCode code="2106-3" displayName="White" codeSystem="2.16.840.1.113883.6.238"
codeSystemName="OMB Standards for Race and Ethnicity"/>
<ethnicGroupCode code="2186-5" displayName="Not Hispanic or Latino"
codeSystem="2.16.840.1.113883.6.238" codeSystemName="OMB Standards for Race and Ethnicity"/>
<languageCommunication>
<languageCode code="eng"/>
<modeCode code="ESP" displayName="Expressed spoken"
codeSystem="2.16.840.1.113883.5.60" codeSystemName="LanguageAbilityMode"/>
<proficiencyLevelCode code="E" displayName="Excellent"
codeSystem="2.16.840.1.113883.5.61" codeSystemName="LanguageAbilityProficiency"/>
<preferenceInd value="true"/>
</languageCommunication>
<languageCommunication>
<languageCode code="ita"/>
<modeCode code="ESP" displayName="Expressed spoken"
codeSystem="2.16.840.1.113883.5.60" codeSystemName="LanguageAbilityMode"/>
<proficiencyLevelCode code="G" displayName="Good"
codeSystem="2.16.840.1.113883.5.61" codeSystemName="LanguageAbilityProficiency"/>
<preferenceInd value="false"/>
</languageCommunication>
</patient>
</patientRole>
</recordTarget>
The US Realm Patient Name template (2.16.840.1.113883.10.20.22.5.1) supports the @use attribute on the name tag. This template also supports the @qualifier attribute on the given and family tags.
These value sets are available in the C-CDA Value Set Release Package available from the Downloads page of the Value Set Authority Center.
Example 10: Logical display order of name pieces
(i.e., such that a receiver which only extracts the text and ignores the markup around <given>, <family>, etc. would still display the name in a way a human would interpret correctly).
<recordTarget>
<!-- Examples of how systems send names and logical display order -->
<!-- Note: This snippet omits other data elements required to meet C-CDA requirements
(telecom, address, etc.) -->
<patientRole>
<id nullFlavor="NI" />
<patient>
<!-- This is okay; "Robin Taylor" is a logical display order -->
<name>
<given>Robin</given>
<family>Taylor</family>
</name>
<!-- This is okay; "Taylor, Robin" implies Lastname, Firstname ordering in the US -->
<name>
<family>Taylor</family>
<delimiter>, </delimiter>
<given>Robin</given>
</name>
<!-- This is okay; "Dr. Robin Taylor" is a logical display order -->
<name>
<prefix qualifier="AC TITLE">Dr. </prefix><!-- Space in element is intentional
(see CDA R2.0 Section 2.23.4) -->
<given>Robin</given>
<family>Taylor</family>
</name>
<!-- This is okay; "Robin Taylor MD" is a logical display order -->
<name>
<given>Robin</given>
<family>Taylor</family>
<suffix qualifier="AC TITLE"> MD</suffix><!-- Space in element is intentional
(see CDA R2.0 Section 2.23.4) -->
</name>
<!-- Don't do this; "Robin Taylor Dr." isn't a logical display order -->
<name>
<given>Robin</given>
<family>Taylor</family>
<prefix qualifier="AC TITLE">Dr. </prefix><!-- Space in element is intentional -->
</name>
<!-- Don't do this; "Taylor Robin" is confusing (is Taylor the given name?) -->
<name>
<family>Taylor</family>
<given>Robin</given>
</name>
</patient>
</patientRole>
</recordTarget>
Patient identifiers are included in a C-CDA within the patientRole structure of the recordTarget. The @extension attribute of the id tag holds the identifier. The @root attribute holds an OID associated with the assigning authority of the identifier. The @assigningAuthorityName attribute holds the name of the assigning authority. Many organizations that assign trusted identifiers exist today and have registered OIDs to support exchange of the identifiers they assign. The table below includes some examples.
| Assigning Authority Name | Assigning Authority OID identifier |
|---|---|
| U.S. Social Security Administration | 2.16.840.1.113883.4.1 |
| Driver’s license issuing authority | 2.16.840.1.113883.12.333 |
| DT.org PATIENT (DirectTrust assigned identity for Consumer entity) | 1.3.6.1.4.1.41179.2.4 |
Table 11: Patient Identifiers
Example 11: How to represent a social security number (SSN)
Some systems may collect a patient’s Social Security number but organizational business rules prohibit distribution of the full number and require that all but the final four digits of the identifier to be masked when distributing this information to data sharing partners.
Content Consumers are strongly encouraged to not treat id elements that include a nullFlavor attribute as globally unique identifiers. |
Example 12: How to represent a social security number (SSN) that has been masked to show only the last four digits
<recordTarget>
<patientRole>
<!-- Example Social Security Number using the root for the Social Security Administration
assigningAuthority. -->
<id assigningAuthorityName="US Social Security Administration" root="2.16.840.1.113883.4.1"
extension="414122222"/>
<!-- SSN provided, but masked to include only the last 4 characters. -->
<!-- Note: both examples are shows for comparison. It would not make sense to use both of
these options in a real C-CDA Document. -->
<!-- When recording SSN in the extension dashes (-) are permitted but not required" -->
<!-- Receiving systems may decide whether to store the last 4 or discard -->
<!-- an id with a nullFlavor SHALL NOT be used for matching or indexing -->
<id assigningAuthorityName="US Social Security Administration" root="2.16.840.1.113883.4.1"
extension="***-**- 2222" nullFlavor="MSK"/>
<addr use="HP">
<!-- HP is "primary home" from codeSystem 2.16.840.1.113883.5.1119 -->
<streetAddressLine>1357 Amber Dr</streetAddressLine>
<city>Beaverton</city>
<state>OR</state>
<postalCode>97006</postalCode>
<country>US</country>
<!-- US is "United States" from ISO 3166-1 Country Codes: 1.0.3166.1 -->
</addr>
<telecom value="tel:+1(555)- 777 - 1234" use="MC"/>
<telecom value="tel:+1(555)- 723 - 1544" use="HP"/>
<!-- HP is "primary home" from HL7 AddressUse 2.16.840.1.113883.5.1119 -->
<patient> </patient>
<providerOrganization> </providerOrganization>
</patientRole>
</recordTarget>
While the recordTarget represents the medical record that this document belongs to, the providerOrganization within the recordTarget represents the provider organization to which the medical record belongs.
C-CDA Content Creators are encouraged to populate the providerOrganization within the recordTarget when the document represents information from a provider’s medical record system. |
C-CDA Content Creators SHOULD include the provider organization's identifier in the id/@extension attribute and SHOULD identify the assigning authority for the identifier using a globally unique identifier (GUID or OID) in the id/@root attribute. [CONF-036] |
CDA includes structures to record the author of information, and separately, the performer of a service. It is important for implementers to avoid recording a performer as an author, if that performer was not the individual who authored content.
A performer participant represents a clinician who actually and principally carried out a service. A performer participation indicated at one location in a document does not conduct throughout the document and must be repeated at each entry to indicate involvement. For example, the performer indicated in the serviceEvent in the CDA header is not automatically implied to be the performer in procedures or medication activities represented by the entries in the document. An author represents the human or machine that authored content. Authors listed in the header are responsible for all content in the document, while authors recorded in a section or entry are only responsible for content within that structure and override the author in the header. Section authorship applies to the full content of a section, including both the narrative block and any entries. Entry authorship applies only to the specific entry.
One example where confusion between these roles might arise for implementers is related to quality measurement use cases, which require clinical documentation to indicate who diagnosed a patient’s condition and when a clinician made or re-confirmed the diagnosis. The provider who documents the diagnosis (data enterer or author) may not be the provider who makes the diagnosis (performer).
The Author Participation template (2.16.840.1.113883.10.20.22.4.119) is used to explicitly indicate an Author in a section or entry. The template provides conformance rules for representing the author and author.time elements associated with individual entries. The HL7 Basic Provenance project also developed additional guidance on sharing the Author in the Provenance - Provenance - Author Participation.
Author Participation
Inclusion of an entry-level author is allowed in open entry templates in C-CDA where use of entry-level authors has not explicitly been prohibited. Its use is required on only one entry template, the Handoff Communication Participants template.
C-CDA Content Creators are encouraged to explicitly indicate the performer for observations and acts, not just the author who documents those activities. |
The example below shows how to identify a practitioner playing the role of author, performer or other roles such as authenticator and legal authenticator. It shows how to represent the provider’s National Provider Identifier (NPI) number and his or her Direct Address. It also shows how to represent a provider Organization’s NPI number and Direct Address. It includes how to indicate the preferred telecom addresses for various forms of communication.
Example 13: How to represent a provider and the provider’s organization in the role of author
This representation pattern is applicable for other roles such as performer, authenticator, legal authenticator, etc. The example shows how to represent NPI information for the provider and for the provider organization. It also shows how to represent Direct address information and how to indicate the preferred telecom address to use.
<!-- an appropriate participation such as author/assignedAuthor -->
<author>
<time value="20180801095245-0400"/>
<assignedAuthor>
<!-- This is a public id where the root is registered to indicate the National Provider ID -->
<id root="2.16.840.1.113883.4.6" extension="1417947383"
assigningAuthorityName="National Provider ID"/>
<!-- This is a public id where the root indicates this is a Provider Direct Address. -->
<!-- root="1.3.6.1.4.1.41179.2.1" is the assigningAuthorityName for
Direct Trust's Covered Entity addresses "DT.org CE" -->
<id root="1.3.6.1.4.1.41179.2.1" extension="rvhs@rvhs.direct.md"
assigningAuthorityName="DT.org CE (Covered Entity)"/>
<addr>
<streetAddressLine>823 Main Street</streetAddressLine>
<city>River Valley</city>
<state>RI</state>
<postalCode>028321</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:+1-(401)539-4321"/>
<!-- The provider's preferred Direct Address is the address identified with his practice. See
below.-->
<telecom use="WP" value="mailto:rvhs@rvhs.direct.md"/>
<assignedPerson>
<name>
<given>Terry</given>
<given>A</given>
<family>Manning</family>
<delimeter>, </delimeter>
<suffix>MD</suffix>
</name>
</assignedPerson>
<representedOrganization>
<id extension="334" root="1.3.6.1.4.1.22812.4.222.334"/>
<!-- This is a public id where the root is registered to indicate the National Provider
ID -->
<!-- Based on context, this is the NPI of the organization -->
<id root="2.16.840.1.113883.4.6" extension="9999999999"
assigningAuthorityName="National Provider ID"/>
<!-- This is a public id where the root indicates this is a Provider Direct Address. -->
<!-- root="1.3.6.1.4.1.41179.2.1" is the assigningAuthorityName for
Direct Trust's Covered Entity addresses "DT.org CE" -->
<id root="1.3.6.1.4.1.41179.2.1" extension="rvhs@rvhs.direct.md"
assigningAuthorityName="DT.org CE (Covered Entity)"/>
<name>River View Health Services</name>
<telecom use="WP" value="tel:+1-(401)539-4321"/>
<telecom use="WP" value="mailto:rvhs@rvhs.direct.md"/>
<addr>
<streetAddressLine>823 Main Street</streetAddressLine>
<city>River Valley</city>
<state>RI</state>
<postalCode>028321</postalCode>
<country>US</country>
</addr>
</representedOrganization>
</assignedAuthor>
</author>
<!-- end of the appropriate role block -->
If a provider’s NPI number is not known, it can be represented using a nullFlavor of UNK. However, is it important to note the id’s populated with a nullFlavor cannot be used as a globally unique identifier.
Example 14: Provider with an unknown NPI number.
<assignedAuthor>
<!-- This is a public id where the root is registered to indicate the National Provider ID -->
<!-- Use nullFlavor UNK if the provider’s NPI number is not known. -->
<id root="2.16.840.1.113883.4.6" nullFlavor="UNK"
assigningAuthorityName="National Provider ID"/>
<!-- This is a public id where the root indicates this is a Provider Direct Address. -->
<!-- root="1.3.6.1.4.1.41179.2.1" is the assigningAuthorityName for
Direct Trust's Covered Entity addresses "DT.org CE" -->
<id root="1.3.6.1.4.1.41179.2.1" extension="rvhs@rvhs.direct.md"
assigningAuthorityName="DT.org CE (Covered Entity)"/>
<addr>
<streetAddressLine>823 Main Street</streetAddressLine>
<city>River Valley</city>
<state>RI</state>
<postalCode>028321</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:+1-(401)539-4321"/>
<!-- The provider's preferred Direct Address is the address identified with his practice. See
below.-->
<telecom use="WP" value="mailto:rvhs@rvhs.direct.md"/>
<assignedPerson>
<name>
<given>Terry</given>
<given>A</given>
<family>Manning</family>
<delimeter>, </delimeter>
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedAuthor>
Every CDA document has exactly one custodian. The custodian represents the organization who is in charge of maintaining the document. The custodian is the steward that is entrusted with the care of the document. The custodian participation satisfies the CDA definition of Stewardship.
Recommendations for care team member representation in the header of documents are included below. This guidance aims to explain the options available for representation of care team members and clarify which care team members belong in the available header structures.
Implementers can help reduce variability in the representation of care team members by adopting these recommendations.
This guide includes representation of care team members in a care team section template with structured entries. The care team section documents care team members involved in support of care provision and coordination for the patient. Business rules are used to determine which care team members are involved in the encounter or services represented and documented in the header. Care team members established in the header should be included in the care team section template as well as any others who are relevant to the patient’s care.
Reference: Care Team Organizer
Because of the variability of how care team members are represented in the header, and because there is a lack of normative guidance on which header items must be rendered, it is recommended that receiving systems should render ALL the participants in the header, rather than only rendering participants of a particular type. The HL7 CDA style sheet supports complete participant rendering.
The term “care team member” encompasses any participant in the care of a patient. A patient’s care team may include individuals providing support to the patient, such as family members or caregivers, as well as physician providers and non-physician providers, including nurses, social workers, behavioral health specialists, community- based providers, technicians, and assistants. In fact, a patient may have more than one care team. Different care teams exist for different purposes and at different times.
When exchanging information about a care team member, it is recommended to capture the name, identification number, and contact information, along with codes to indicate the type of provider and the role they play on the patient’s care team. These details help to distinguish care team members from different care settings and helps clarify who is involved in the patient’s care and in what ways.
Within CDA, care team members are represented as participants in elements of the document header and may be associated with the patient (i.e. guardian), the clinical encounter, and/or service event(s) detailed in the document, and the document itself. Applicable header elements for documenting care team members from Chapter 1.1 of the C- CDA Implementation Guide are described in the following table.
| Participant | Description |
|---|---|
| documentationOf/ serviceEvent/ performer |
Care team member who performs the service event detailed in the document. Examples: PCP, surgeon, consulting physician |
| encompassingEncounter/ responsibleParty |
Care team member who is responsible for the patient encounter described in the document. Examples: PCP, surgeon, consulting physician, home health nurse |
| recordTarget | The patient, his or herself, is considered a member of the Care team. |
| author | Care team member who documents events included in the documentation of the encounter or documentation of the provision of care over time. Example: PCP, surgeon, consulting physician, home health nurse |
| custodian | The steward of the document is likely an organization with a role on the patient’s care team, although not necessarily. Example: PCP practice, Hospital, home health agency, HIE |
| legalAuthenticator | Care team member who authenticates content contained in the document and accepts legal responsibility. Examples: PCP, consulting physician, attending physician |
| authenticator | Care team member who authenticates content contained in the document. Examples: PCP, consulting physician, attending physician |
| informationRecipient | Care team member who the document is intended for. Examples: PCP, caregiver, consulting physician |
| participant | Other supporting care team members associated with the patient. Examples: Caregiver, family member, emergency contact |
Table 12: Header Elements for Care Team Members
In the header of an Encounter Summary, a care team member may be documented as fulfilling more than one responsibility. For example, a consulting physician who sees a patient in a clinical encounter may be:
The physician also may be the person who legally authenticates the information contained in the document. In this example, the consulting physician is participating as the encompassingEncounter/responsibleParty, the author, the authenticator and the legalAuthenticator.
In the header of a Patient Summary, it is most common to see care team members identified in the documentationOf/serviceEvent as performers who were involved in the provision of care for the patient during the range of time covered by the summary. Again, depending on the use case behind how the Patient Summary is generated, care team members also may be documented in other roles such as the author, the authenticator and the legalAuthenticator.
Semantically, the actRelationships hold the key data elements for documenting Care Team members in the header. These are the data elements that tell who was responsible for the patient encounter and who was responsible for performing care services.
For Encounter Summary documents, C-CDA Content Creators are strongly encouraged to document the provider who is responsible for the encounter in the componentOf/encompassingEncounter/responsibleParty. |
For Patient Summary documents, C-CDA Content Creators are strongly encouraged to document providers who played the role of Primary Care Provider for the patient during the range of time covered in the summary using the documentationOf/serviceEvent/performer. |
Sharing clinical documents with payers is one use case for C-CDA. To meet the needs of this use case, document authentication and signing of C-CDA documents guidance is provided.
The legalAuthenticator is recorded in ClinicalDocument.legalAuthenticator and represents a participant who has legally authenticated the document. Authenticators are recorded in ClinicalDocument.authenticator, and each authenticator represents a participant who has attested to the accuracy of the document, but who does not have privileges to legally authenticate the document. An example would be a resident physician who sees a patient and dictates a note and signs it. Later an attending physician may sign it who would be reprensented by the legalAuthenticator participant.
Additional Guidance is in the following table:
All documents SHALL have a legalAuthenticator. In the case of generated summary documents, institutions may meet the requirement for a Legal Authenticator by extending the practice of maintaining a “Signature on File”. The person, entity, organization, role, etc. indicated is responsible for the contents of the note where it is understood that this Legal Authenticator is not an author of the document. Policy for determining who is responsible for legal authentication of the summary document rests with the originating organization. In real-world exchange, organizations and roles are being used as legalAuthenticators; this is consistent with the CDA base standard. [AIGEX-HD4] |
All documents that contain sdtc:signatureText, SHALL conform to the HL7 Digital Signatures and Delegation of Rights Implementation Guide R1. [AIGEX-HD7] |
The HL7 Digital Signatures and Delegation of Rights Implementation Guide R1 defines the standards for digitally signing C-CDA documents.
C-CDA Content Creators are encouraged to include the sdtc:signatureText extension for Authenticators and LegalAuthenticators. |
C-CDA Content Consumers are encouraged to render certificate information associated with digital signed C-CDA Documents when rendering the document. |
Two common use cases for C-CDA are exchanging Patient Summary Dcouments and Exchanging Encounter Summary Documents.
In a Patient Summary, the documentationOf/serviceEvent holds the key information about the range of time covered by the document and the key providers involved in the provision of the summarized care.
In an Encounter Summary, the header needs to capture the context of care for a particular encounter via the encompasssingEncounter.
For more information and Guidance see Concise Consolidated CDA: Deploying Encounter Summary CDA Documents with Clinical Notes .
In an Encounter Summary, C-CDA Content Creators are encouraged to populate the documentationOf/serviceEvent, including the serviceEvent/effectiveTime, serviceEvent/code, and serviceEvent/performer(s). |
In a Patient Summary, C-CDA Content Creators are strongly encouraged to populate the documentationOf/serviceEvent based on conformance requirements of the C-CDA specification for CCD documents. |
If an Encounter occurred as a result of a previously placed order (e.g., a visit to perform an MRI), the id of that order should be provided as context for the Encounter.
In an Encounter Summary, C-CDA Content Creators are encouraged to populate the infulfillmentOf/order and the infulfillmentOf/order/id element where the order/id references the id of a previously placed order. |
The Order Fulfilled area of the header is encouraged to carry information about fulfilled orders, rereferrals or other requests that have been fulfilled. |
The ParentDocument represents the source of a document revision, addendum, or transformation. The relatedDocument/parentDocument/id element is a mechanism used to link a revised, addendum, or transformed document to its original source document. There are many situations where a document may be updated. For example, a pending laboratory result or a missing note may trigger an update. The base CDA standard provides this mechanism to replace or append a previously sent document through the parentDocument relationship.
C-CDA Content Creators cannot know with certainty what documents a Content Consumer has previously stored. Therefore, it always is preferable for Content Creators to send a complete document that replaces a prior document rather than sending an addendum to a prior document. Indicate the parent document is being replaced by using a replaceRelationship type of RPLC.
Example 15: Replacement Discharge Summary Document with Hospital Course Added
<ClinicalDocument> <realmCode code="US">
<typeId root="2.16.840.1.113883.1.3" extension="POCD_HD000040"/>
<templateId root="2.16.840.1.113883.10.20.22.1.1" extension="2015-08- 01" >
<templateId root="2.16.840.1.113883.10.20.22.1.8" extension="2015-08- 01" >
<id root="2.16.840.1.113883.19.5.99999.1" extension="20160414145050">
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC" code="18842-5"
displayName="Discharge Summary" />
<title>Health Summary</title>
<effectiveTime value="20160414145050-0500">
<confidentialityCode codeSystem="2.16.840.1.113883.5.25" code="N">
<languageCode code="en-US">
<setId extension="20160414014447" root="2.16.840.1.113883.19.5.99999.19">
<versionNumber value="2">
<relatedDocument typeCode="RPLC">
<parentDocument> <id root="2.16.840.1.113883.19.5.99999.1" extension="20160414014447">
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC" code="18842-5"
displayName="Discharge Summary">
<setId extension="20160414014447" root="2.16.840.1.113883.19.5.99999.19">
<versionNumber value="1"> </parentDocument>
</relatedDocument>
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.5"/>
<code code="8648-8" displayName="HOSPITAL COURSE" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<title>Hospital Course</title>
<text>The patient was admitted
</text>
While all C-CDA documents share a common US Realm Header template, the C-CDA implementation guide defines distinct types of document templates for exchanging different types of clinical notes.
Document-level templates describe the purpose and rules for constructing a conforming CDA document. Document templates include constraints on the CDA header and indicate contained section-level templates.
Each document-level template contains the following information:
Templates for structured documents (those that include a structuredBody) also define required and optional section-level templates.
All section-level templates include human readable narrative that conveys the information in that section. CDA calls this the Narrative Block. Each section-level template also includes:
The section may also define required and optional entry-level templates. Entry-level templates are structures for representing the information in the Narrative Block using machine representations that facilitates computerized information processing.
The table below describes the various document types defined by C-CDA for use in representing clinical notes as structured documents that facilitate digital information exchange.
| Document-Level Templates for Representing Clinical Notes as Structured Documents | Description |
|---|---|
| Care Plan | A Care Plan (including Home Health Plan of Care [HHPoC]) is a consensus-driven dynamic plan that represents a patient’s and Care Team Members’ prioritized concerns, goals, and planned interventions. The CDA Care Plan represents an instance of this dynamic Care Plan at a point in time. The CDA document itself is NOT dynamic. |
| Consultation Note | The Consultation Note is generated by a request from a clinician for an opinion or advice from another clinician. |
| Continuity of Care Document (CCD) | The Continuity of Care Document (CCD) represents a core data set of the most relevant administrative, demographic, and clinical information facts about a patient's healthcare, covering one or more healthcare encounters. It provides a means for one healthcare practitioner, system, or setting to aggregate all of the pertinent data about a patient and forward it to another to support the continuity of care. |
| Discharge Summary | The Discharge Summary is a document which synopsizes a patient's admission to a hospital, LTPAC provider, or other setting. It provides information for the continuation of care following discharge. |
| History and Physical | A History and Physical (H&P) Note is a medical report that documents the current and past conditions of the patient. It contains essential information that helps determine an individual's health status. |
| Operative Note | The Operative Note is a frequently used type of procedure note with specific requirements set forth by regulatory agencies. The Operative Note is created immediately following a surgical or other high-risk procedure. It records the pre- and post-surgical diagnosis, pertinent events of the procedure, as well as the condition of the patient following the procedure. |
| Progress Note | This template represents a patient’s clinical status during a hospitalization, outpatient visit, treatment with a LTPAC provider, or other healthcare encounter. |
| Procedure Note | This template encompasses many types of non-operative procedures including interventional cardiology, gastrointestinal endoscopy, osteopathic manipulation, and many other specialty fields. Procedure Notes are differentiated from Operative Notes because they do not involve incision or excision as the primary act. |
| Referral Note | A Referral Note communicates pertinent information from a provider who is requesting services of another provider of clinical or non-clinical services. |
| Transfer Summary | The Transfer Summary standardizes critical information for exchange of information between providers of care when a patient moves between health care settings. |
Table 13: C-CDA Document-Level Templates for Representing Clinical Notes as Structured Documents
The table below describes the document template defined by C-CDA for use in representing clinical notes as an unstructured document that facilitates digital information exchange.
| Document Templates for Unstructured Clinical Notes | Description |
|---|---|
| Unstructured Document | An Unstructured Document (UD) document type can (1) include unstructured content, such as a graphic, directly in a text element with a mediaType attribute, or (2) reference a single document file, such as a word-processing document using a text/reference element. |
Table 14: Document Templates of Unstructured Clinical Notes
The C-CDA Unstructured Document template defines a C-CDA document with a structured header and a non-XML body. This template is used to convey information as an embedded object or referenced file. It may be appropriate for use cases where the exchange of clinical information does not require structured representation of the content. Simply having the structured header information may be beneficial for information exchange.
When the content to be exchanged does not have structured document templates defined in C-CDA, such as for Laboratory Report Narrative or Pathology Report Narrative, the Unstructured Document template can be used to represent the clinical note information.
C-CDA Content Creators are encouraged to support the Unstructured Document template for representing clinical note types when the type of clinical note to be exchanged does not have an appropriate structured document template or when it is not possible for the C-CDA Content Creator to represent the clinical note information using a structured document template. |
Each distinct document template defines the specific code that is used to identify that document type. Since the ClinicalDocument.code is defined as a CE, it allows for translation codes to also be specified. These translation codes can provide more details about the document type.
A Care Plan is a consensus-driven dynamic plan that represents a patient’s and Care Team Members’ prioritized concerns, goals, and planned interventions. It serves as a blueprint shared by all Care Team Members (including the patient, their caregivers and providers), to guide the patient’s care. A Care Plan integrates multiple interventions proposed by multiple providers and disciplines for multiple conditions.
A Care Plan represents one or more Care Plans and serves to reconcile and resolve conflicts between the various Care Plans developed for a specific patient by different providers. Care Plans include the patient’s life goals and require Care Team Members (including patients) to prioritize goals and interventions, the reconciliation process becomes more complex as the number of merged Care Plans increase. The Care Plan also serves to enable longitudinal coordination of care.
The C-CDA Care Plan represents an instance of a dynamic Care Plan at a point in time. The CDA document itself is NOT dynamic.
This document template enables Care Plan information to be shared in a way that includes:
A Care Plan document can include entry references from the information in these sections to the information (entries) in other sections.
The table below describes the required and optional sections in a Care Plan document template: Care Plan
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Care Plan | Health Concerns Section Goals Section |
Activities Section Health Status Evaluations and Outcomes Section |
| Sample | CarePlan.xml Sample | Included with this Companion Guide |
Table 16: Care Plan: Document Template.
The Consultation Note is generated by a request from a clinician for an opinion or advice from another clinician. Consultations may involve face-to-face time with the patient or may fall under the auspices of telemedicine visits. Consultations may occur while the patient is inpatient or ambulatory. The Consultation Note should also be used to summarize an Emergency Room or Urgent Care encounter. A Consultation Note includes the reason for the referral, history of present illness, physical examination, and decision-making components (Assessment and Plan).
The table below describes the required and optional sections in a Consultation Note document template:Consultation Note
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Consultation Note | History of Present Illness Section Allergies and Intolerances Section Problem Section |
Assessment Section Assessment and Plan Section Plan of Treatment Section Reason for Visit Section Physical Exam Section Chief Complaint Section Chief Complaint and Reason for Visit Section Family History Section General Status Section Past Medical History Immunizations Section Medications Section Procedures Section Results Section Social History Section Vital Signs Section Functional Status Section Review of Systems Section Medical Equipment Section |
Table 17: Consultation Note: Document Template.
The Continuity of Care Document (CCD) represents a core data set of the most relevant administrative, demographic, and clinical information facts about a patient's healthcare, covering one or more healthcare encounters. It provides a means for one healthcare practitioner, system, or setting to aggregate all of the pertinent data about a patient and forward it to another to support the continuity of care. The primary use case for the CCD is to provide a snapshot in time containing the germane clinical, demographic, and administrative data for a specific patient. The key characteristic of a CCD is that the ServiceEvent is constrained to "PCPR" “care provision.” This means that the contents of the document reflect the care that was actually provided within the time range indicated in serviceEvent.effectiveTime range. It reports on care that has already been provided. The CCD provides a historical tally of the care over a range of time and is not a record of new services delivered. More specific use cases, such as a Discharge Summary, Transfer Summary, Referral Note, Consultation Note, or Progress Note, are available as alternative documents in this guide.
The table below describes the required and optional sections in a Continuity of Care Document (CCD) document template: Continuity of Care Document (CCD)
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Continuity of Care Document (CCD) | Allergies and Intolerances Section Medications Section Problem Section Results Section Social History Section Vital Signs Section |
Procedures Section Encounters Section Family History Section Functional Status Section Immunizations Section Medical Equipment Section Payers Section Plan of Treatment Section Mental Status Section Nutrition Section Advance Directives Section Goals Section Health Concerns Section |
Table 18: Continuity of Care Document: Document Template.
The Discharge Summary is a document which summarizes a patient's admission to a hospital, LTPAC provider, or other setting. It provides information for the continuation of care following discharge. The Joint Commission requires the following information to be included in the Discharge Summary:
The best practice for a Discharge Summary is to include the discharge disposition in the display of the header.
The table below describes the required and optional sections in a Discharge Summary document template: Discharge Summary
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Discharge Summary | Allergies and Intolerances Section Hospital Course Section Discharge Diagnosis Section Plan of Treatment Section (DIR) |
Discharge Medications Section Chief Complaint Section Chief Complaint and Reason for Visit Section Nutrition Section Family History Section Functional Status Section Past Medical History History of Present Illness Section Admission Diagnosis Section Admission Medications Section Hospital Consultations Section Hospital Discharge Instructions Section Hospital Discharge Studies Summary Section Immunizations Section Procedures Section Reason for Visit Section Review of Systems Section Social History Section Vital Signs Section Discharge Medications Section Goals Section Health Concerns Section |
| Example | DischargeSummary.xml Sample | Included with this Companion Guide |
Table 20: Discharge Summary: Document Template.
A History and Physical (H&P) Note is a medical report that documents the current and past conditions of the patient. It contains essential information that helps determine an individual's health status. The first portion of the report is a current collection of organized information unique to an individual. This is typically supplied by the patient or the caregiver, concerning the current medical problem or the reason for the patient encounter. This information is followed by a description of any past or ongoing medical issues, including current medications and allergies. Information is also obtained about the patient's lifestyle, habits, and diseases among family members. The next portion of the report contains information obtained by physically examining the patient and gathering diagnostic information in the form of laboratory tests, imaging, or other diagnostic procedures. The report ends with the clinician's assessment of the patient's situation and the intended plan to address those issues. A History and Physical Examination is required upon hospital admission as well as before operative procedures. An initial evaluation in an ambulatory setting is often documented in the form of an H&P Note.
The table below describes the required and optional sections in a History and Physical document template: History and Physical
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| History and Physical | Allergies and Intolerances Section Family History Section General Status Section Past Medical History Medications Section Physical Exam Section Results Section Review of Systems Section Social History Section Vital Signs Section |
Assessment Section Plan of Treatment Section Assessment and Plan Section Chief Complaint Section Chief Complaint and Reason for Visit Section History of Present Illness Section Immunizations Section Instructions Section Problem Section Procedures Section Reason for Visit Section |
Table 21: History and Physical: Document Template.
The Operative Note is a frequently used type of procedure note with specific requirements set forth by regulatory agencies. The Operative Note is created immediately following a surgical or other high-risk procedure. It records the pre- and post-surgical diagnosis, pertinent events of the procedure, as well as the condition of the patient following the procedure. The report should be sufficiently detailed to support the diagnoses, justify the treatment, document the course of the procedure, and provide continuity of care.
The table below describes the required and optional sections in an Operative Note document template: Operative Note
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Operative Note | Anesthesia Section Complications Section Preoperative Diagnosis Section Procedure Estimated Blood Loss Section Procedure Findings Section Procedure Specimens Taken Section Procedure Description Section Postoperative Diagnosis Section |
Procedure Implants Section Operative Note Fluids Section Operative Note Surgical Procedure Section Plan of Treatment Section Planned Procedure Section Procedure Disposition Section Procedure Indications Section Surgical Drains Section |
Table 22 Operative Note: Document Template.
A Procedure Note encompasses many types of non-operative procedures including interventional cardiology, gastrointestinal endoscopy, osteopathic manipulation, and many other specialty fields. Procedure Notes are differentiated from Operative Notes because they do not involve incision or excision as the primary act. The Procedure Note is created immediately following a non-operative procedure. It records the indications for the procedure and, when applicable, post-procedure diagnosis, pertinent events of the procedure, and the patient’s tolerance for the procedure. It should be detailed enough to justify the procedure, describe the course of the procedure, and provide continuity of care.
The table below describes the required and optional sections in Procedure Note
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Procedure Note | Complications Section Procedure Description Section Procedure Indications Section Postprocedure Diagnosis Section |
Assessment Section Assessment and Plan Section Plan of Treatment Section Allergies and Intolerances Section Anesthesia Section Chief Complaint Section Chief Complaint and Reason for Visit Section Family History Section Past Medical History History of Present Illness Section Medical (General) History Section Medications Section Medications Administered Section Physical Exam Section Planned Procedure Section Procedure Disposition Section Procedure Estimated Blood Loss Section Procedure Findings Section Procedure Implants Section Procedure Specimens Taken Section Procedures Section Reason for Visit Section Review of Systems Section Social History Section |
Table 23: Procedure Note: Document Template.
The Progress Note represents a patient’s clinical status during a hospitalization, outpatient visit, treatment with a LTPAC provider, or other healthcare encounter. Taber’s medical dictionary defines a Progress Note as “An ongoing record of a patient's illness and treatment. Physicians, nurses, consultants, and therapists record their notes concerning the progress or lack of progress made by the patient between the time of the previous note and the most recent note. A Progress Note is not a re-evaluation note. A Progress Note is not intended to be a Progress Report for Medicare. Medicare B Section 1833(e) defines the requirements of a Medicare Progress Report. The Joint Document Content Work Group recommends use of the Progress Note document template to represent an encounter summary for a non-inpatient setting in Chapter 2.2 Outpatient/Ambulatory Summary (Progress Note Document). The Progress Note document template does not include any required sections and the open nature of the template enables Content Creators to include the right sections to express the source data or the needed sections to satisfy the requirements of Content Consumers.
The table below describes the required and optional sections in a Progress Note document template: Progress Note
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Progress Note | Assessment Section Plan of Treatment Section Assessment and Plan Section Allergies and Intolerances Section Chief Complaint Section Activities Section Instructions Section Medications Section Objective Section Physical Exam Section Problem Section Results Section Review of Systems Section Subjective Section Vital Signs Section Nutrition Section |
|
| Example | ProgressNote.xml Sample | Included with this Companion Guide |
Table 24 Progress Note: Document Template.
A Referral Note communicates pertinent information from a provider who is requesting services of another provider of clinical or non-clinical services which includes the reason for the referral and additional information that would augment decision making and care delivery. Examples of referral situations include when a patient is:
The table below describes the required and optional sections in a Referral Note document template: Referral Note
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Referral Note | Problem Section Allergies and Intolerances Section Medications Section Reason for Referral Section |
Plan of Treatment Section History of Present Illness Section Family History Section Immunizations Section Procedures Section Results Section Review of Systems Section Social History Section Vital Signs Section Functional Status Section Physical Exam Section Nutrition Section Mental Status Section Medical Equipment Section Assessment Section Assessment and Plan Section Past Medical History General Status Section Advance Directives Section Health Concerns Section Goals Section |
| Example | ReferralNote.xml Sample | Included with this Companion Guide |
Table 25: Referral Note: Document Template.
The Transfer Summary template describes constraints on the Clinical Document Architecture (CDA) header and body elements for a Transfer Summary. The Transfer Summary standardizes critical information for exchange of information between providers of care when a patient moves between health care settings. Standardization of information used in this form will promote interoperability; create information suitable for reuse in quality measurement, public health, research, and for reimbursement.
The table below describes the required and optional sections in a Transfer Summary document template: Transfer Summary
| Document Type | Required Sections | Optional Sections |
|---|---|---|
| Transfer Summary | Allergies and Intolerances Section Medications Section Problem Section Results Section Vital Signs Section Reason for Referral Section |
Physical Exam Section Encounters Section Family History Section Functional Status Section Discharge Diagnosis Section Immunizations Section Medical Equipment Section Payers Section Plan of Treatment Section Procedures Section Social History Section Mental Status Section General Status Section Review of Systems Section Nutrition Section Past Medical History History of Present Illness Section Assessment and Plan Section Assessment Section Admission Medications Section Admission Diagnosis Section Course of Care Section |
Table 26: Transfer Summary: Document Template.
Implementers may use templates developed in other CDA implementation guides which are compatible with C- CDA. Employing additional C-CDA compatible templates within existing document types will expand the range of interoperable information available for exchange and help address emerging use cases for data exchange.
C-CDA Content Creators are encouraged to include in existing C-CDA documents, other C-CDA compatible section- level templates defined in other implementation guides. |
A C-CDA compatible template is a template that further constrains a template defined in C- CDA or a template that does not conflict with templates defined in C-CDA. Determining if a template is C-CDA compliant may require human discernment and consensus building within the C-CDA implementer community. A particularly important collection of C-CDA compatible templates are the Supplemental Implementation Guides, which define new template versions and templates for additional use cases. These Supplemental Implementation Guides are published within the main C-CDA specification Product Brief page
C-CDA Content Creators are encouraged to support further constraints on the document templates defined in C-CDA |
The idea of improving the communication of information contained in clinical notes has been around for decades. Long before C-CDA, a documentation methodology called “SOAP (Subjective, Objective, Assessment, and Plan) notes” was invented. The birth of the problem-oriented medical record (POMR) and SOAP note marked an epoch in the history of health care. Dr. Lawrence Weed, developer of the SOAP note and professor of medicine and pharmacology at Yale University, challenged conventional medical documentation and advocated for a scientific structure to frame clinical reasoning in the 1950s. Today, the SOAP note is the most common method of documentation used by providers to input notes into patients’ medical records. They allow providers to record and share information in a universal, systematic and easy to read format. Ineffective communication contributes to the top causes of sentinel events and continues to be an unremitting area for refinement.
After the development of the HL7 Clinical Document Architecture R2 standard in 2005, an alliance of healthcare vendors, providers and associations pooled resources in a rapid-development initiative. In a span of three years, the Health Story Project produced eight Health Level Seven (HL7) data standards for the flow of information using common types of healthcare documents. The alliance examined thousands of common clinical notes generated by a variety of medical transcription solutions and identified what structured sections were needed to represent the SOAP notes commonly generated by current-day clinicians. The “Health Story Guides,” as they were originally named, defined the initial set of section templates based on this analysis. Every section template includes a section.text element designed to hold the human readable narrative of that section of the structured document.
While no longer active today, the HIMSS Health Story Project provided education to the health IT community on tools and resources to aid in the creation of comprehensive electronic records that tell a patient's complete health story. Some especially educational concepts from this initiative are summarized in the following chapters.
This roundtable presentation explained the twelve common clinical document types that are defined in Consolidated CDA (C-CDA) and described how each can be leveraged for information exchange in different care settings and for different encounter types. When should each C-CDA clinical document type be leveraged? What sections are used in C-CDA clinical documents and how do they differ from a Continuity of Care Document (CCD)? The Roundtable showed how to apply the SOAP framework to reveal the storytelling power of C-CDA by thinking of the section templates in terms of the subjective, objective, assessment, and plan information each contains. In some cases, the information in one section may be classified in multiple SOAP categories. The categorization is an approximation designed to improve understanding of the full collection of C-CDA section templates.
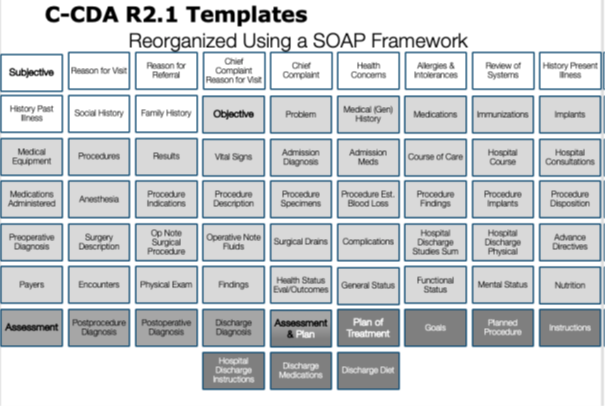 |
Visualizing C-CDA section templates by applying the SOAP framework
This presentation explained how documentation-based exchange via Consolidated Clinical Document Architecture (C-CDA), when implemented correctly, has the power to capture and share a more comprehensive electronic record that can be used to improve care.
By 2011, when C-CDA R1.0 was first published, a larger industry effort brought together CDA templates for clinical SOAP notes that had been defined by several organizations including Integrating the Healthcare Enterprise (IHE), HITSP, and HL7. The harmonized work included not only definitions for section templates needed to structure common clinical note types, but also included entry templates that defined how to represent the human readable section information using machine processable “clinical statements”. Clinical statements were templated using the syntax and data structures supported by the HL7 CDA R2 standard which derived its data types and modeling from an Version 2.0.7 of the V3 RIM found here HL7 V3 Reference Information Model (RIM). The HL7 V3 RIM is the cornerstone of all HL7 Version 3 standards. It is a shared model between all domains and, as such, forms a common basis from which all domains can create information exchange artifacts and messages.
Today in C-CDA and in the C-CDA Supplemental Implementation Guides, the C-CDA standards development community has defined and published a wide array of section templates to represent clinical information in the context of structured documents. It is important to note that the purpose of each section template is dependent on the context of its intended use within a larger document structure. While some templates have been defined generically and are suitable for reuse in multiple structured documents, others due to the nature of their definitions may not be appropriate for re-use across other documents. For this reason, is it important for the context of the overarching document to be considered when determining if it is appropriate to include a particular type of section template in a particular type of document template.
C-CDA Content Creators are encouraged to use section templates that are appropriate within the context of a document based on the defined purpose of the section template. |
The following guidance elements are not specific to any one C-CDA template but rather are overarching guidance elements that apply to an entire C-CDA document.
The author role is key to understanding the provenance of the information.
The roles populated in the header of the document apply to each section of content as well, unless explicitly indicated otherwise. If the author information for a section is not explicitly declared, then the author of the information in that section can be assumed to be the author contained in the document header. This is behavior within a CDA document is called context conduction.
This assumption extends to entries contained in the section as well. If the author information for an entry is not explicitly declared, then the author of the information in that entry can be assumed to be the author contained in the encompassing section.
While it is generally preferred that provenance be conveyed either at the document level, or at the entry level, it is possible for provenance to be conveyed at the section level when the default context conduction does not apply.
When representing a clinical note using structured sections, each section of information receives its context from the document’s header. If author information for a section is not explicitly declared, then the author of that section of information can be assumed to be the same as the author information contained in the document header.
The recordTarget, author, and informant roles populated in the header of the document apply to each section of content as well, unless explicitly indicated otherwise. The author role is key to understanding the provenance of the information in the document. If the author information for a section is not explicitly declared, then the author of that section of information can be assumed to be the same as the author information contained in the header.
This assumption extends to entries contained in the section as well, unless the author information is explicitly declared at the entry level. While it is generally preferred that provenance be conveyed at the entry level, provenance information included at the document or section level conducts to the entry when provenance at the entry level is not stated explicitly.
Template conformance may be declared at any level of a C-CDA document—header, section, entry, or within an entry at a sub-structural level.
A template declaration in a C-CDA section asserts the constraints applicable for that section of XML. The template declaration tells validators and Content Consumers what to expect in terms of the information that, may, should, or shall be populated within this section of the document.
C- CDA templates are identified with a templateId. The templateId is a two-part identifier that consists of a root and an extension. The root identifies the named template and the extension identifies the version of that template. Initially C-CDA templates did not include versions. The templateId/@extension attribute was not used. Many of those original template versions are still used in C-CDA today. A duplicate templateId declaration is a template declaration for the same structural part of a C-CDA document (i.e., document, section, entry, entry-part) with identical @root and @extension information. The order of the @root and @extension attributes does not matter when determining duplication.
To avoid confusion and minimize inclusion of unnecessary information in C-CDA documents, implementers should avoid including duplicate or irrelevant templateId declarations.
C-CDA Content Creators are encouraged to not declare conformance to irrelevant templates. |
As standards evolve an implementer community may decide to deprecate a version of a template or a collection of templates published in a version of an implementation guide. It should be anticipated that implementer communities may determine that a certain version of a template should not be used going forward.
C-CDA Content Creators and C-CDA Content Consumers are encouraged to not attribute semantic meaning to templateId declarations in C-CDA documents. |
C-CDA Content Creators are encouraged to support template deprecation requiring discontinued use of all versions of a templateId or a specific version of a templateId. |
Example 16: Declaring template conformance at the section level.
<!-- Allergies and Intolerances Section-->
<component>
<section>
<!-- Conformant to C-CDA Allergies and Intolerances Section-->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<!-- Conformant to C-CDA R1.1 Allergies and Intolerances Section -->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<id root="0937FF9A-00CE-11E6-B4C5-0050568B000B"/>
<code code="48765-2" displayName="Allergies &or adverse reactions Doc"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Allergies</title>
<text>
</text>
</section>
</component>
Example 17: Declaring conformance to multiple templates.
<!—Substance or Device Allergy -->
<templateId root="2.16.840.1.113883.10.20.24.3.90" extension="2014-06- 09" />
<templateId root="2.16.840.1.113883.10.20.24.3.90"/>
<!— Allergy – Intolerance Observation -->
<templateId root="2.16.840.1.113883.10.20.22.4.7" extension="2014-06- 09" />
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
Example 18: (Wrong) Duplicate template declarations at the section level.
<!-- Allergies and Intolerances Section-->
<component>
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId extension="2015-08- 01" root="2.16.840.1.113883.10.20.22.2.6.1" />
<id root="0937FF9A-00CE-11E6-B4C5-0050568B000B"/>
<code code="48765-2" displayName="Allergies &or adverse reactions Doc"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Allergies</title>
<text>
</text>
</section>
</component>
Example of how to include templateIds for Companion Guide templates
<observation classCode="OBS" moodCode="EVN">
<!-- ** Problem Observation ** -->
<!-- Problem Observation (C-CDA 1.1) -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" />
<!-- Problem Observation (C-CDA 2.1) -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2015-08- 01" />
<!-- Problem Observation (C-CDA Companion Guide R3) -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2022- 06 - 01" />
<id root="ab1791b0-5c71-11db-b0de-0800200c9a66" />
...
</observation>
The CDA requirement for human readability guarantees that a receiver of a CDA document can algorithmically display the clinical content of the note on a standard Web browser. This requirement impacts C-CDA in the following ways:
These principles and requirements have led to the current approach, where the material to be rendered is placed into the section.text field. In some cases, the data design of the entry templates required in the section heavily influences what clinical information can be expected to be present in the Narrative Block. In other cases, where entry templates are optional or not defined at all, the content in the Narrative Block may reflect information gathered in source systems as text and human crafted notes.
If the CDA Body is structured, the Content Creator includes the attested narrative content in the appropriate section.text field, regardless of whether information is also conveyed in CDA entries. An originator of a CDA document is not required to fully encode all narrative into CDA entries within the CDA body. Within specific implementations, trading partners may ascribe additional originator responsibilities to create various entries that meet certain conformance requirements or meet the conformance requirements described by defined templates.
Example 19: Sample Narrative Block in a Section
<section>
...
<text>
<table>
<colgroup>
<col width="25%"/>
<col width="25%"/>
<col width="25%"/>
<col width="25%"/>
</colgroup>
<thead>
<tr>
<th>Information Type</th>
<th>Date</th>
<th>Relevant Information</th>
<th>Documented By</th>
</tr>
</thead>
<tbody>
<tr ID="SocialHistory_1">
<td>Tobacco smoking status:</td>
<td><content>(04/12/2016)</content></td>
<td><content>Never smoked</content></td>
<td><content>M.Smith</content> <content>(04/12/2016)</content></td>
</tr>
</tbody>
</table>
</text>
...
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.78"/>
<templateId root="2.16.840.1.113883.10.20.22.4.78"
extension="2014-06- 09"/>
<id extension="64020-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.1040.1"/>
<code code="72166-2" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Tobacco smoking status"/>
<text><reference value="#SocialHistory_1"></reference></text>
<statusCode code="completed"/>
<effectiveTime value="20160412"/>
<value xsi:type="CD" code="266919005" codeSystem="2.16.840.1.113883.6.96"
displayName="Never smoked tobacco" />
<author>
<time value="20160412"/>
<assignedAuthor>
<id extension="10.1" root="1.2.840.114350.1.1"/>
</assignedAuthor>
</author>
</observation>
</entry>
...
</section>
CDA permits additional information conveyed in the document that is there primarily for machine processing that is not authenticated and need not be rendered. However, the requirement that all attested content be present in the section.text field, suggests best practice is for all clinical content to be rendered through section.text.
C-CDA Content Creators are encouraged to include all clinical content conveyed by a document in the section.text field. |
The content model of the CDA Narrative Block schema is specially hand crafted to meet the requirements outlined above. The table below provides information on some of the formatting tags commonly used to organize and render the human readable information in the Narrative Block.
| Tag Description | Description |
|---|---|
| <content> | The CDA <content> element is used to wrap a string of text so that it can be explicitly referenced, or so that it can suggest rendering characteristics. The <content> element contains an optional identifier, that can serve as the target of a reference. All values of attributes of type XML id must be unique within the document (per the W3C XML specification. The originalText component of a RIM attribute present in any CDA entry can make explicit reference to the identifier, thereby indicating the original text associated with the attribute in the CDA entry. |
| <br> | The CDA element is used to indicate a hard line break. It differs from the CDA <paragraph> element in that the element has no content. Receivers are required to interpret this element when rendering so as to represent a line break. |
| <list> | A CDA <list> is similar to the HTML list. A CDA <list> has an optional caption and contains one or more <item> elements. A CDA <item> element contains an optional caption, which if present must come first before any other character data. The required listType attribute specifies whether the <list> is ordered or unordered (with unordered being the default). Unordered lists are typically rendered with bullets, whereas ordered lists are typically rendered with numbers, although this is not a requirement. |
| <table> | The CDA <table> is similar to the HTML table. The table markup is for presentation purposes only and, unlike a database table, does not possess meaningful field names. |
| <linkHtml> | The CDA <linkHtml> is a generic referencing mechanism, similar, but not identical, to the HTML anchor tag. It can be used to reference identifiers that are either internal or external to the document. |
| styleCode attribute | The styleCode attribute is used within the CDA Narrative Block to give the instance author the ability to suggest rendering characteristics of the nested character data (e.g. Bold, Underline, Italics). Receivers are not required to render documents using the style hints provided and can present stylized text in accordance with their local style conventions. Reference: HL7 CDA Chapter 4.3.5.11 for information on additional stylecodes. |
Table 27: Tags for formatting Narrative Text
Sharing documents requires different views based on user needs. HL7 Relevant and Pertinent Survey highlights the need to enhance CDA document rendering. CDA uses XML stylesheets with @styleCode, as defined by IHE's MCV Profile. Refer to IHE Multiple Content Views (MCV) Profile for details and examples.
Implementers should use MCV Profile's @styleCode values for consistency in rendering human-readable content, covering codes, dates, dateTimes, alerts, and more. These values enhance clinical content rendering features, ensuring information is not omitted or obscured unless a specific rendering view calls for it.
Temporal information is often included as relevant and pertinent information to be exchanged. However, implementers need to be diligent when representing date/time information to make sure irrelevant or inaccurate levels of precision are not introduced when representing this type of information.
CDA features prioritize template reusability and flexibility for tailored content in sections, aligning with patient, provider, or setting needs. Most C-CDA templates are open, allowing additional relevant content. Example: The Social History Section focuses on data influencing a patient's health (e.g., smoking, pregnancy). Information like education, housing, food security, or transportation can be added here if not covered elsewhere. Implementers can also explore external CDA templates from Supplemental Implementation Guides for expanded use cases within the C-CDA standard. C-CDA Specification Product Brief. Caution: Implementers should be aware that recipients may not understand templates included at an unexpected location within a document.
To indicate the application of business rules limiting information in a C-CDA section, Content Creators should clarify the time range in the section's human-readable text. For example, the Vital Signs Section includes recent vital signs based on business rules, like the most recent readings within a requested time interval, documented in the section. The Section Time Range Observation template is helpful when responding to queries requesting extensive data. It allows specifying the business logic for data constraint within a section. This template communicates the 'business logic' used to restrict information to a specific time range. For instance, if a CCD document request covers 5 years but the system only returns one year of past lab results, the Section Time Range template indicates this limitation. The business logic is stated in the value element using datatype IVL_TS. No effectiveTime element is included.
Example 20: Example of Section Time Range Observation
<section>
...
<title>Procedures</title>
<text>
<content ID="Proc_STR">Procedures performed between 08/15/2012 and 08/15/2015.</content>
...
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.201" extension="2016-06- 01"/>
<code code="82607-3" codeSystem="2.16.840.1.113883.6.1"
displayName="Section Date and Time Range"/>
<text>
<reference value="#Proc_STR"/>
</text>
<statusCode code="completed"/>
<value xsi:type="IVL_TS">
<low value="20120815"/>
<high value="20150815"/>
</value>
</observation>
</entry>
</section>
As explained in the Health Story Roundtable presentation titled The Storytelling Power of C-CDA, understanding the purpose of the C-CDA section templates is not facilitated by considering them in alphabetical order. The C-CDA Implementation Guide presents them in alphabetical order to speed access for readers. Considering the C-CDA section templates using the SOAP framework makes it easier to see how these sections can be used in a structured document to express the content of a clinical SOAP note.
The application of the SOAP framework does not produce a perfect classification result. Some sections don’t fit well into the SOAP framework. They have been classified as “other types of sections”. Some sections are defined to contain “heterogeneous” information, meaning the section’s content spans the boundaries of the SOAP framework. For example, the Assessment and Plan Section contains both assessment (A) and plan (P) information. Section structures that contain “homogeneous” information, all of the same type with the same purpose, improves information processing.
C-CDA Content Creators are encouraged to use section templates containing “homogeneous” information with regards to not mixing subjective, objective, assessment, and plan types of information together in a single section. |
| Section Name LOINC OID |
Purpose Description | |
|---|---|---|
| Subjective Section 61150-9 2.16.840.1.113883.10.20.21.2.2 |
This section describes in a narrative format the patient’s current condition and/or interval changes as reported by the patient or by the patient’s guardian or another informant. | |
| Reason for Visit 29299-5 2.16.840.1.113883.10.20.22.2.12 |
This section records the patient’s reason for the patients’ visit (as documented by the provider). Local policy determines whether Reason for Visit and Chief Complaint are in separate or combined sections. | |
| Reason for Referral 42349-1 1.3.6.1.4.1.19376.1.5.3.1.3.1:2014-06-09 |
This section describes the clinical reason why a provider is sending a patient to another provider for care. The reason for referral may become the reason for visit documented by the receiving provider. | |
| Chief Complaint 10154-3 2.16.840.1.113883.10.20.22.2.13 |
This section records the patient’s chief complaint (the patient’s own description). | |
| Chief Complain and Reason for Visit 46239-0 2.16.840.1.113883.10.20.22.2.13 |
This section records the patient’s chief complaint (the patient’s own description) and/or the reason for the patient’s visit (the provider’s description of the reason for visit). Local policy determines whether the information is divided into two sections or recorded in one section serving both purposes. |
|
| Health Concerns Section 75310-3 2.16.840.1.113883.10.20.22.2.58:2015-08-01 |
This section contains data describing an interest or worry about a health state or process that could possibly require attention, intervention, or management. A Health Concern is a health-related matter that is of interest, importance or worry to someone, who may be the patient, patient’s family or patient’s health care provider. Health concerns are derived from a variety of sources within IEHR. Health concerns can be medical, surgical, nursing, allied health or patient-reported concerns. “Transportation difficulties” for someone who doesn't drive and has trouble getting to appointments, or “Underinsured” for someone who doesn't have sufficient insurance to properly cover their medical needs such as medications. Problem Concerns are a subset of Health Concerns that have risen to the level of importance that they typically would be described in the Problems Section. |
|
| Allergies and Intolerances Section 48765-2 2.16.840.1.113883.10.20.22.2.6.1:2015-08-01 |
This section lists and describes any medication allergies, adverse reactions, idiosyncratic reactions, anaphylaxis/anaphylactoid reactions to food items, and metabolic variations or adverse reactions/allergies to other substances (such as latex, iodine, tape adhesives). At a minimum, it should list currently active and any relevant historical allergies and adverse reactions. | |
| Review of Systems Section 10187-3 1.3.6.1.4.1.19376.1.5.3.1.3.18 |
This section contains a relevant collection of symptoms and functions systematically gathered by a clinician. It includes symptoms the patient is currently experiencing, some of which were not elicited during the history of present illness, as well as a potentially large number of pertinent negatives, for example, symptoms that the patient denied experiencing. | |
| History of Present Illness 10164-2 1.3.6.1.4.1.19376.1.5.3.1.3.4 |
This section describes the history related to the reason for the encounter. It contains the historical details leading up to and pertaining to the patient’s current complaint or reason for seeking medical care. | |
| Past Medical History 11348-0 2.16.840.1.113883.10.20.22.2.20:2015-08-01 |
This section contains a record of the patient’s past complaints, problems, and diagnoses. It contains data from the patient’s past, up to the patient’s current complaint or reason for seeking medical care. | |
| Social History Section 29762-2 2.16.840.1.113883.10.20.22.2.17:2015-08-01 |
This section contains social history data that influence a patient’s physical, psychological or emotional health (e.g., smoking status, pregnancy, work). Demographic data, such as marital status, race, ethnicity, and religious affiliation, is captured in the header. | |
| Family History Section 10157-6 2.16.840.1.113883.10.20.22.2.15:2015-08-01 |
This section contains data defining the patient’s genetic relatives in terms of possible or relevant health risk factors that have a potential impact on the patient’s healthcare risk profile. |
Table 28: Subjective Information
| Sections defined in C-CDA LOINC OID |
Purpose Description |
|---|---|
| Objective Section 61149-1 2.16.840.1.113883.10.20.21.2.1 |
This section contains data about the patient gathered through tests, measures, or observations that produce a quantified or categorized result. It includes important and relevant positive and negative test results, physical findings, review of systems, and other measurements and observations. |
| Problems Section 11450-4 2.16.840.1.113883.10.20.22.2.5.1:2015-08-01 |
This section lists and describes all relevant clinical problems at the time the document is generated. At a minimum, all pertinent current and historical problems should be listed. Overall health status may be represented in this section. |
| Medical (General) History Section 11329-0 2.16.840.1.113883.10.20.22.2.39 |
This section describes all aspects of the medical history of the patient even if not pertinent to the current procedure, and may include chief complaint, past medical history, social history, family history, surgical or procedure history, medication history, and other history information. The history may be limited to information pertinent to the current procedure or may be more comprehensive. The history may be reported as a collection of random clinical statements or it may be reported categorically. Categorical report formats may be divided into multiple subsections including Past Medical History, Social History. |
| Medications Section 10160-0 2.16.840.1.113883.10.20.22.2.1.1:2014-06-09 |
This section contains a patient’s current medications and pertinent medication history. At a minimum, the currently active medications are listed. An entire medication history is an option. The section can describe a patient’s prescription and dispense history and information about intended drug monitoring. |
| Immunizations Section 11369-6 2.16.840.1.113883.10.20.22.2.2.1:2015-08-01 |
This section defines a patient’s current immunization status and pertinent immunization history. The primary use case for the Immunization Section is to enable communication of a patient’s immunization status. The section should include current immunization status and may contain the entire immunization history that is relevant to the period of time being summarized. |
| Medical Equipment Section 46264-8 2.16.840.1.113883.10.20.22.2.23:2014-06-09 |
This section defines a patient’s implanted and external health and medical devices and equipment. This section lists any pertinent durable medical equipment (DME) used to help maintain the patient’s health status. All equipment relevant to the diagnosis, care, or treatment of a patient should be included. Devices applied to, or placed in, the patient are represented with the Procedure Activity Procedure template. Equipment supplied to the patient (e.g., pumps, inhalers, wheelchairs) is represented by the Non-Medicinal Supply Activity template. These devices may be grouped together within a Medical Equipment Organizer. |
| Procedures Section 47519-4 2.16.840.1.113883.10.20.22.2.7.1:2014-06-09 |
This section describes all interventional, surgical, diagnostic, or therapeutic procedures or treatments pertinent to the patient historically at the time the document is generated. The section should include notable procedures. |
| Results Section 30954-2 2.16.840.1.113883.10.20.22.2.3.1:2015-08-01 |
This section contains the results of observations generated by laboratories, imaging and other procedures. The scope includes observations of hematology, chemistry, serology, virology, toxicology, microbiology, plain x-ray, ultrasound, CT, MRI, angiography, echocardiography, nuclear medicine, pathology, and procedure observations. This section often includes notable results such as abnormal values or relevant trends. |
| Vital Signs Section 8716-3 2.16.840.1.113883.10.20.22.2.4.1:2015-08-01 |
This section contains relevant vital signs for the context and use case of the document type, such as blood pressure, heart rate, respiratory rate, height, weight, body mass index, head circumference, pulse oximetry, temperature, and body surface area. The section should include notable vital signs such as the most recent, maximum and/or minimum, baseline, or relevant trends. Vital signs are represented in the same way as other results. However, they are represented in their own section to follow clinical conventions. |
| Course of Care Section 8648-8 2.16.840.1.113883.10.20.22.2.64 |
This section describes what happened during the course of an encounter. |
| General Status Section 10210-3 2.16.840.1.113883.10.20.2.5 |
This section describes general observations and readily observable attributes of the patient, including affect and demeanor, apparent age compared to actual age, gender, ethnicity, nutritional status based on appearance, body build and habitus (e.g., muscular, cachectic, obese), developmental or other deformities, gait and mobility, personal hygiene, evidence of distress, and voice quality and speech. |
| Functional Status Section 47420-5 2.16.840.1.113883.10.20.22.2.14:2014-06-09 |
This section contains observations and assessments of a patient’s physical abilities. A patient’s functional status may include information regarding the patient’s ability to perform Activities of Daily Living (ADLs) in areas such as Mobility (e.g., ambulation), Self-Care (e.g., bathing, dressing, feeding, grooming) or Instrumental Activities of Daily Living (IADLs) (e.g., shopping, using a telephone, balancing a check book). Problems that impact function (e.g., dyspnea, dysphagia) can be contained in the section. |
| Mental Status Section 10190-7 2.16.840.1.113883.10.20.22.2.56:2015-08-01 |
This section contains observations and evaluations related to a patient’s psychological and mental competency and deficits including, but not limited to any of the following types of information: • Appearance (e.g., unusual grooming, clothing or body modifications) • Attitude (e.g., cooperative, guarded, hostile) • Behavior/psychomotor (e.g., abnormal movements, eye contact, tics) • Mood and affect (e.g., anxious, angry, euphoric) • Speech and Language (e.g., pressured speech, perseveration) • Thought process (e.g., logic, coherence) • Thought content (e.g., delusions, phobias) • Perception (e.g., voices, hallucinations) • Cognition (e.g., memory, alertness/consciousness, attention, orientation) – which were included in Cognitive Status Observation in earlier publications of C-CDA.• Insight and judgment (e.g., understanding of condition, decision making) |
| Nutrition Section 61144-2 2.16.840.1.113883.10.20.22.2.57 |
This section represents diet and nutrition information including special diet requirements and restrictions (e.g., texture modified diet, liquids only, enteral feeding). It also represents the overall nutritional status of the patient and nutrition assessment findings. |
| Specific to Inpatient Encounter Notes | |
| Admission Diagnosis Section 46241-6 2.16.840.1.113883.10.20.22.2.43:2015-08-01 |
This section contains a narrative description of the problems or diagnoses identified by the clinician at the time of the patient’s admission. This section may contain a coded entry which represents the admitting diagnoses. |
| Admission Medications Section 42346-7 2.16.840.1.113883.10.20.22.2.44:2015-08-01 |
This section contains the medications taken by the patient prior to and at the time of admission to the facility. |
| Hospital Course Section 8648-8 1.3.6.1.4.1.19376.1.5.3.1.3.5 |
This section describes the sequence of events from admission to discharge in a hospital facility. |
| Hospital Consultations Section 18841-7 2.16.840.1.113883.10.20.22.2.42 |
This section records consultations that occurred during the admission. |
| Hospital Discharge Studies Summary Section 11493-4 2.16.840.1.113883.10.20.22.2.16 |
This section records the results of observations generated by laboratories, imaging procedures, and other procedures. The scope includes hematology, chemistry, serology, virology, toxicology, microbiology, plain x-ray, ultrasound, CT, MRI, angiography, echocardiography, nuclear medicine, pathology, and procedure observations. This section often includes notable results such as abnormal values or relevant trends and could record all results for the period of time being documented. |
| Hospital Discharge Physical Section 10184-0 1.3.6.1.4.1.19376.1.5.3.1.3.2 |
This section records a narrative description of the patient’s physical findings generated by the discharge physician at the time of discharge. |
| Discharge Medications Section 10183-2 2.16.840.1.113883.10.20.22.2.11.1:2015-08-01 |
This section contains the medications the patient is intended to take or stop after discharge. Current, active medications must be listed. The section may also include a patient’s prescription history and indicate the source of the medication list. |
| Specific to Procedure and Operative Notes | |
| Medications Administered Section 29549-3 2.16.840.1.113883.10.20.22.2.38:2014-06-09 |
This section usually resides inside a Procedure Note describing a procedure. This section defines medications and fluids administered during the procedure, its related encounter, or other procedure related activity excluding anesthetic medications. |
| Anesthesia Section 59774-0 2.16.840.1.113883.10.20.22.2.25:2014-06-09 |
This section records the type of anesthesia (e.g., general or local) and may state the actual agent used. This may be a subsection of the Procedure Description Section. The full details of anesthesia are usually found in a separate Anesthesia Note. |
| Procedure Indications Section 59768-2 2.16.840.1.113883.10.20.22.2.29:2014-06-09 |
This section contains the reason(s) for the procedure or surgery. This section may include the pre-procedure diagnoses as well as symptoms contributing to the reason for the procedure. |
| Medical Equipment Section 46264-8 urn:hl7ii:2.16.840.1.113883.10.20.22.2.23:2014-06-09 |
This section contains devices that have been placed in a patient. This section is also relevant for recording information about non-implanted medical equipment and non-medicinal supplied equipment (e.g. wheelchair, hearing aid, walker). |
| Complications Section 55109-3 2.16.840.1.113883.10.20.22.2.37:2015-08-01 |
This section contains problems that occurred during or around the time of a procedure. The complications may be known risks or unanticipated problems. |
Table 29: Objective Information
| Sections defined in C-CDA LOINC OID |
Purpose Description |
|---|---|
| Assessment Section 51848-0 2.16.840.1.113883.10.20.22.2.8 |
This section (also referred to as “impression” or “diagnoses” outside of the context of CDA) represents the clinician’s conclusions and working assumptions that will guide treatment of the patient. The assessment may be a list of specific disease entities or a narrative block. |
| Assessment and Plan Section 51847-2 2.16.840.1.113883.10.20.22.2.9:2014-06-09 |
This section represents the clinician’s conclusions and working assumptions that will guide treatment of the patient. The Assessment and Plan Section may be combined or separated to meet local policy requirements. Best practice is to separate these distinct types of information by using the Assessment Section: templateId 2.16.840.1.113883.10.20.22.2.8 and the Plan of Treatment Section: templateId 2.16.840.1.113883.10.20.22.2.10:2014-06-09 |
| Specific to Inpatient Encounter Notes: | |
| Discharge Diagnosis Section 11535-2 2.16.840.1.113883.10.20.22.2.24:2015-08-01 |
This section represents problems or diagnoses present at the time of discharge which occurred during the hospitalization. This section includes an optional entry to record patient diagnoses specific to this visit. Problems that need ongoing tracking should also be included in the Problem Section. |
| Specific to Procedure and Operative Notes: | |
| Postprocedure Diagnosis Section 59769-0 2.16.840.1.113883.10.20.22.2.36:2015-08-01 |
This section records the diagnosis or diagnoses discovered or confirmed during the procedure. Often it is the same as the preprocedure diagnosis or indication. |
| Postoperative Diagnosis Section 10219-4 2.16.840.1.113883.10.20.22.2.35 |
This section records the diagnosis or diagnoses discovered or confirmed during the surgery. Often it is the same as the preoperative diagnosis. |
Table 30: Assessment Information
| Sections defined in C-CDA LOINC OID |
Purpose Description |
|---|---|
| Goals Section 61146-7 2.16.840.1.113883.10.20.22.2.60 |
This section represents patient Goals. A goal is a defined outcome or condition to be achieved in the process of patient care. Goals include patient-defined over-arching goals and health concern-specific or intervention-specific goals to achieve desired outcomes. |
| Advance Directives Section 42348-3 2.16.840.1.113883.10.20.22.2.21.1:2015-08-01 |
This section contains data defining the patient’s advance directives and any reference to supporting documentation, including living wills, healthcare proxies, and CPR and resuscitation status. If the referenced documents are available, they can be included in the exchange package. |
| Assessment and Plan Section 51847-2 2.16.840.1.113883.10.20.22.2.9:2014-06-09 |
This section represents the clinician’s conclusions and working assumptions that will guide treatment of the patient. The Assessment and Plan Section may be combined or separated to meet local policy requirements. Best practice is to separate these distinct types of information by using the Assessment Section: templateId 2.16.840.1.113883.10.20.22.2.8 and the Plan of Treatment Section: templateId 2.16.840.1.113883.10.20.22.2.10:2014-06-09 |
| Plan of Treatment Section 18776-5 2.16.840.1.113883.10.20.22.2.10:2014-06-09 |
This section, formerly known as "Plan of Care", contains data that define pending orders, interventions, encounters, services, and procedures for the patient. It is limited to prospective, unfulfilled, or incomplete orders and requests only. All active, incomplete, or pending orders, appointments, referrals, procedures, services, or any other pending event of clinical significance to the current care of the patient should be listed. |
| Instructions Sections 69730-0 2.16.840.1.113883.10.20.22.4.20:2014-06-09 |
This section can be used in several ways, such as to record patient instructions within a Medication Activity or to record fill instructions within a supply order. |
| Specific to Procedure and Operative Notes: | |
| Planned Procedures Section 59772-4 2.16.840.1.113883.10.20.22.2.30:2014-06-09 |
This section contains the procedure(s) that a clinician planned based on the preoperative assessment. |
| Specific to Procedure and Operative Notes: | |
| Hospital Discharge Instructions Section 8653-8 2.16.840.1.113883.10.20.22.2.41 |
This section records instructions at discharge. |
Table 31: Plan/Planning Information
| Sections defined in C-CDA LOINC OID |
Purpose Description |
|---|---|
| Encounters Section 46240-8 2.16.840.1.113883.10.20.22.2.22.1:2015-08-01 2.16.840.1.113883.10.20.22.2.18:2015-08-01 |
This section is relevant in nearly all documents. It lists and describes any healthcare encounters pertinent to the patient’s current health status or historical health history. An encounter is an interaction, regardless of the setting, between a patient and a practitioner who is vested with primary responsibility for diagnosing, evaluating, or treating the patient’s condition. It may include visits, appointments, or non-face-to-face interactions. An encounter also may be a contact between a patient and a practitioner who has primary responsibility (exercising independent judgment) for assessing and treating the patient at a given contact. This section may include a single encounter in an Encounter Summary. It contains relevant encounters for the time period being summarized in a Patient Summary Document. |
| Payers Section 48768-6 2.16.840.1.113883.10.20.22.2.18:2015-08-01 |
This section contains data on the patient’s payers, "third party" insurance, self-pay, other payer or guarantor, or some combination of payers, and is used to define which entity is the responsible fiduciary for the financial aspects of a patient’s care. Each unique instance of a payer and all the pertinent data needed to contact, bill to, and collect from that payer should be included. Authorization information that can be used to define pertinent referral, authorization tracking number, procedure, therapy, intervention, device, or similar authorizations for the patient or provider, or both should be included. At a minimum, the patient’s pertinent current payment sources should be listed. |
Table 32: Other Information
Several section templates have been developed specifically to supplement C-CDA. They are defined within the
context of C-CDA Supplemental Guides that have been balloted and reconciled separately from C-CDA, and are
published alongside the main C-CDA Specification
https://www.hl7.org/implement/standards/product_brief.cfm?product_id=492. These guides are developed with
the intention of eventually replacing earlier version of the templates in C-CDA or eventually being added to the set
of templates considered a part of the C-CDA set of templates.
At the time of this publication, the following Supplemental Implementation Guides are published within the C-CDA specification Product Brief page and may be used within a C-CDA 3.0 Templates:
The guidance below pertains when document sections include machine processable discrete data to aid processing of information contained in the section. The following general entry-level guidance is applicable to all C-CDA entry templates.
Best practice for CDA creation is to represent all human readable text in the section, then reference the text from the discrete entries that represent the human readable information as machine processable data. To include narrative text linking, the text element of the primary (outer-most) act in an entry should point, by reference, to the portion of the narrative text corresponding to the meaning of the entire clinical statement expressed in the discrete entry.
Example 21: Narrative Text with Links to Machine Processable Data
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.7.1" extension="2014-06- 09" />
<code code="47519-4" displayName="Procedures" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" />
<title>Procedures</title>
<text>
<table border="1" width="100%">
<thead>
<tr>
<th>Procedure Name</th>
<th>Code</th>
<th>CodeSystem</th>
<th>Target Site</th>
<th>Date of Procedure</th>
</tr>
</thead>
<tbody>
<tr id=PROCEDURESUMMARY_1>
<td id=PROCEDURE_1>Skin care: graft site</td>
<td>406177009</td>
<td>SNOMED CT</td>
<td>11207009 (Structure of right thigh)</td>
<td>2015-06- 23</td>
</tr>
</tbody>
</table>
</text>
<entry typeCode="DRIV">
<procedure classCode="PROC" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.14" extension="2014-06- 09" />
<id root="93ad269d-40a6-4d71-bcc6-6978598820d9" />
<code code="406177009" displayName="Skin care: graft site"
codeSystem="2.16.840.1.113883.6.96">
<originalText>
<reference value="#PROCEDURE_1" />
</originalText>
</code>
<text><!--<reference value="#PROCEDURESUMMARY_1" />--></text>
<statusCode code="completed" />
<effectiveTime value="20150623" />
<methodCode nullFlavor="UNK" />
<targetSiteCode code="11207009" displayName="Structure of right thigh"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT" />
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119" />
<time value="20150623" />
<assignedAuthor>
<id extension="db659e09-b0b0-4e51-a6e9-7eb5abdae4e4" />
<code nullFlavor="UNK" />
<assignedPerson>
<name nullFlavor="NI" />
</assignedPerson>
</assignedAuthor>
</author>
</procedure>
</entry>
</section>
In accordance with general CDA principles for human readability, every CDA shall be viewable through the use of a CDA stylesheet. it is recommended to regularly test using the HL7 CDA stylesheet approved by SDWG and managed in the HL7 GitHub.
When a CDA document section contains coded discrete entries (such as allergy, medication, problem, etc. to support machine processing of the available human readable information), coded data within the discrete entry SHOULDD include an originalText element to link the coded information back to the original human readable information represented by that code. The use of code/originalText/reference and value/originalText/reference should be used where it is useful to point to the human readable information associated with more specific areas within the narrative related to a specific coded element within a discrete entry.
Narrative text linking from the text element in the entry (for the entire discrete entry, meaning the entire machine processable clinical statement) and from the originalText element (for a specific part of the clinical statement) referencing from coded concepts in the entry can be used together to provide very tight correspondences between human readable and machine processable information.
The referencing mechanism when used with the original text component of a coded CDA entry:
Sometimes the original text will be repeated in the originalText element rather than using a reference link into the narrative text. This is not incorrect and should not be flagged as an error. In this case, the originalText element, allows the human readable information to include a quality check.
The HL7 CDA standard recommends use of narrative text linking to minimize mismatch errors where the human readable narrative information is not identical to the original text.
Example 22: originalText used to record the term actually selected from the EHR
<code code="9999123"
displayName="Obsessional thoughts of augmented reality video
games" codeSystem="2.16.840.1.113883.6.96">
<originalText><reference value="#Obsessive thoughts related to video
games"/></originalText>
</code>
Example 23: originalText linking the coded concept used in the machine entry to the narrative
originalText linking the coded concept used in the machine entry to the narrative that was actually
stated by the clinician or what was actually in the EHR when this problem was entered by the clinician.
Narrative text linking is the preferred method of representation because it eliminates the possibility for
mismatch between the attested content in the section.text and what was actually seen or said by the
clinician.
<code code=" 9999123 "
displayName="Obsessional thoughts of augmented reality video
games" codeSystem="2.16.840.1.113883.6.96">
<originalText><reference value="#Problem_1"/></originalText>
</code>
The originalText contains what the human stated or the terms selected from the EHR user interface.
NOTE: The C-CDA specification does not currently include an explicit coded indicator to define whether the narrative text contains additional information beyond the coded data or not. Narrative text and user selected terms may routinely have more robust content than the structured entries. The narrative text may contain additional nuances and should never be ignored by receiving systems.
The originalText attribute is encouraged to reference text in the Narrative Block which mirrors what the clinician saw or selected in the user interface of the system that created the source data. |
It is valuable to send the local code that represents the originalText as a translation, along with one or more translations to publicly defined code systems. This practice is encouraged in FHIR when using data elements of type CodableConcept. In CDA this can be accomplished through translationCode.
The following best practices were agreed upon by HL7 Structured Documents Work Group and the HL7 Vocabulary Work Group in January of 2017 :
Example 24: TranslationCode, with originalText and local coded term
<value xsi:type="CD" code="254838004" displayName="Carcinoma of Breast"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT" >
<originalText>
<reference value="#problem1"></reference>Carcinoma of right breast, stage 2, estrogen
receptor
positive</originalText>
<!-- User Selected Term Coding -->
<translation code="40780512" displayName="Carcinoma of right breast, stage 2, estrogen
receptor
positive" codeSystem="2.16.840.1.113883.3.247.1.1"
codeSystemName="EHRorInterfaceTerminologyCodeSystem"/>
<!-- “Secondary" Codes -->
<translation code="174.9" codeSystem="2.16.840.1.113883.6.103" codeSystemName="ICD-9CM"
displayName="Malignant neoplasm of breast (female), unspecified site"/>
<translation code="C50.911" codeSystem="2.16.840.1.113883.6.90" codeSystemName="ICD-10- CM"
displayName="Malignant neoplasm of unspecified site of right female breast"/>
<translation code="416053008" codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"
displayName="Estrogen receptor positive tumor"/>
</value>
When sending coded information, the CD datatype (most commonly used in <code> and <value> elements) has a ‘displayName’ element. This element is intended to be a valid human readable representation of the concept defined by the code system and associated with the ‘code’ element at the time of data entry. As an example, for LOINC codes, the ‘displayName’ element should convey either the short name or long name in LOINC for the code used in the associated code element.
The displayName attribute is strongly encouraged not to modify the meaning of the code. Note: Testability requires fuzzy match capabilities. |
The guidance for the use of the displayName element are:
The displayName attribute is encouraged to be included where syntactically allowed. |
When a CDA document includes coded data in discrete entries (such as allergen, medication, problem, etc.) to support machine processing, every discrete entry SHOULD include a text element that references the human readable representation of the information discretely represented by a code.
For example, a new version of SNOMED CT is released with a new problem code of 99999123 and a display name of “Obsessional thoughts of augmented reality video games” and this code is used in a Problem Observation. If neither originalText nor display were included in the xml entry, the human readable narrative for the entry (if not otherwise processed) might only say, “Problem 99999123 began on July 6, 2016 as noted by Dr. Ishihara.” This would violate the core CDA principal of human readability.
C-CDA Content Creators are encouraged to include a human readable representation of the concept associated with the code as defined by the code system in the @displayName attribute of a code element. |
C-CDA Content Validators is encouraged to require a fuzzy match between the @displayName attribute of a code element and the preferred concept description for the code as defined by the associated code system. |
Example 25: Code Display Name Representation
<code code=" 9999123 "
displayName="Obsessional thoughts of augmented reality video
games" codeSystem="2.16.840.1.113883.6.96">
<originalText><reference value="#PROBLEM1"/></originalText>
</code>
The "id" element serves as a globally unique identifier for data collections, such as documents, sections, entries, or sub-entries (e.g., authors).
Consistency in using ids is crucial within a document and across different document instances from the same system. If data remains the same, the id remains constant. Any changes leading to a new instance of data result in a new id.
For instance, if a patient's penicillin allergy is documented in a CCD, the id for this allergy should remain the same in subsequent C-CDA documents unless there's a fundamental change. Consistent ids simplify data reconciliation and matching, increasing accuracy.
One way to implement consistent unique ids is by maintaining multiple GUIDs for each object in a database. Object Identifiers (OIDs) can identify the assigning authority for each GUID. CDA id elements consist of a root (GUID or OID) and an optional extension, ensuring global uniqueness.
Vendors have flexibility in generating valid GUIDs.
Content Creators are encouraged to include ids to identify pieces of information and use consistent ids for the same piece of information |
HL7 V3 CDA handles unavailable, unknown, or incomplete data with 'null flavors,' representing coded values that explain the missing information. Assigning a value for missing data is required for validation and considered good practice in other cases. Precision in indicating null flavors is encouraged to convey the reason for missing data. The CDA R2 standard's null flavor vocabulary provides a complete hierarchy of these values. The @nullFlavor attribute is especially informative when used with intervals. For instance, in a Tobacco Use observation, "effectiveTime/high/@nullFlavor='UNK'" indicates the patient has stopped using a tobacco product, but the exact cessation time is unknown. Other nullFlavors like NI (no information), NAV (not available), and NASK (not asked) convey uncertainty about the patient's current substance use.
Example 26: Tobacco Use – Current Smoker with an unknown stop date
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.78"/>
<templateId root="2.16.840.1.113883.10.20.22.4.78" extension="2014-06- 09"/>
<id extension="64020-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.1040.1"/>
<code code="72166-2" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Tobacco smoking status"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20100412"/>
<high nullFlavor="UNK"/>
</effectiveTime>
<value code="77176002" codeSystem="2.16.840.1.113883.6.96" displayName="Smoker"
xsi:type="CD"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"/>
</observation>
Example 27: Tobacco Use – Smoker where cessation date was not asked
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.78"/>
<templateId root="2.16.840.1.113883.10.20.22.4.78" extension="2014-06- 09"/>
<id extension="64020-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.1040.1"/>
<code code="72166-2" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Tobacco smoking status"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20100412"/>
<high nullFlavor="NASK"/>
</effectiveTime>
<value code="77176002" codeSystem="2.16.840.1.113883.6.96" displayName="Smoker"
xsi:type="CD"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"/>
</observation>
If the resolution to a problem is not known, its effectiveTime/high should contain a value or nullFlavor=UNK. If the nullFlavor=NA, then the problem is definitely not resolved. And if the nullFlavor is anything else, then it is unclear as to whether the problem is still active or if it has been resolved.
The @nullFlavor attribute also conveys when information is unknown. However, a nullFlavor SHALL NOT be used to bypass IG requirements for convenience ( e.g. you may send a nullFlavor=UNK for a patient’s birthTime when it is not recorded in a chart, but you must not send it simply because it is too difficult to convert the method your system uses to record birth dates to an HL7 timestamp). NullFlavor attributes need not be included for non- required elements, such as religiousAffiliationCode. If an element is optional and unknown, it may simply be omitted.
The following guidelines clarify the use of the “No Information” nullflavor=”NI” pattern for a section with no information:
If a document template requires a section to be present and the source system contains no information to populate the section:
The section is encouraged to be omitted or included and declared as having no information. |
Machine-readable data in these sections is essential for clinical best practice and should not be omitted unless the entire section contains no information (section/@nullFlavor=NI). In such cases, you may use "unknown" information within specific acts, like Procedure Activity, along with a description and a code for the nature of the unknown data.
The goal is to describe unknown information explicitly for accurate communication. Fields related to problems, medications, and medication allergies cannot be left blank; instead, the section must be included with a null value, and the narrative text should explicitly state that the information is unknown.
Example 28: No Information Problems Section
<!-- ************************* PROBLEM LIST ****************************** -->
<component>
<!-- nullFlavor of NI indicates No Information.-->
<section nullFlavor="NI">
<!-- conforms to Problems section -->
<templateId root="2.16.840.1.113883.10.20.22.2.5.1" extension="2015-08- 01"/>
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="PROBLEM LIST"/>
<title>PROBLEMS</title>
<text>No Information</text>
</section>
</component>
Distinguishing between "no information" and "no known information" is important. In the case of "no known information," the author isn't asserting the presence or absence of data for the element. "No information" explicitly states that there's no data for that element in the system. For example, "I don't know if the patient has any allergies" (no information) versus "The patient claims no allergies" (no known). When asserting "no known" information, use negation indicators (negationInd). Avoid explicit codes like "no known allergies"; instead, use a negation indicator on the act, accompanied by a text description and a code indicating the data lacks value. Acknowledging some ambiguity due to earlier RIM versions in CDA R2, specific examples like "no known problems" and "no known allergies" have been adopted.
Example 29: Allergy List
<!-- ************************* ALLERGY LIST ****************************** -->
<component>
<section>
<!-- Allergies section-->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<code code="48765-2" codeSystem="2.16.840.1.113883.6.1"/>
<title>ALLERGIES, ADVERSE REACTIONS, ALERTS</title>
<text>No Known Allergies</text>
<entry typeCode="DRIV">
<!-- Allergy Concern Act -->
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.30" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<id root="36e3e930-7b14-11db-9fe1-0800200c9a66"/>
<!-- SDWG supports 48765-2 or CONC in the code element -->
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<statusCode code="active"/> <!--currently tracked concerns are active concerns-->
<effectiveTime>
<low value="20091201"/> <!--show time when the concern first began being tracked-->
</effectiveTime>
<entryRelationship typeCode="SUBJ">
<!-- No Known Allergies -->
<!-- The negationInd = true negates the observation/value -->
<!-- The use of negationInd corresponds with the newer Observation.valueNegationInd
-->
<observation classCode="OBS" moodCode="EVN" negationInd="true">
<!-- allergy - intolerance observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7" extension="2014-06- 09"/>
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<id root="4adc1020-7b14-11db-9fe1-0800200c9a66"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<!-- N/A - author/time records when this assertion was made -->
<effectiveTime>
<low nullFlavor= "NA" />
</effectiveTime>
<value xsi:type="CD" code="419199007"
displayName="Allergy to substance (disorder)"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"/>
<author>
<time value="20100103"/>
<assignedAuthor>
<id extension="99999999" root="2.16.840.1.113883.4.6"/>
<code code="200000000X" codeSystem="2.16.840.1.113883.6.101"
displayName="Allopathic & Osteopathic Physicians"/>
<telecom use="WP" value="tel:555-555-1002"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
<!-- In C-CDA R2.1 the participant is required. -->
<participant typeCode="CSM">
<participantRole classCode="MANU">
<playingEntity classCode="MMAT">
<code nullFlavor="NA"/>
</playingEntity>
</participantRole>
</participant>
</observation>
</entryRelationship>
</act>
</entry>
To indicate a section has "no information," include the section with a null value of NI. To show that a section contains information in the source system but is excluded from the exchange document, include the section with a null value of MSK, indicating that the sender has not provided this information due to security, privacy, or other reasons. The specific wording for "no information" and "masked information" in the narrative text can be determined locally.
Example 30: Allergies Section with No Information
<!-- ************************* ALLERGY LIST ****************************** -->
<component>
<!-- nullFlavor of NI indicates No Information.-->
<section nullFlavor="NI">
<!-- Allergies section-->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<code code="48765-2" codeSystem="2.16.840.1.113883.6.1"/>
<title>ALLERGIES, ADVERSE REACTIONS, ALERTS</title>
<text>No Information</text>
</section>
Example 31: Excluding section due to business rules.
Entire section excluded because business rules of the author determine the section of information is not present due to security, privacy, or other reasons.
<section nullFlavor="MSK">
<templateId root="2.16.840.1.113883.10.20.22.2.7.1" extension="2014-06- 09"/>
<id root="4536582C-018F-11E6-9EF4-0050568B1D1B"/>
<code code="47519-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="History of procedures"/>
<title>Procedures - from Last 3 Months</title>
<text>
Information has not been provided by the sender due to security, privacy, or other reasons.
There may be an alternate mechanism for gaining access to this information.
</text>
</section>
Example 32: Procedures Section with Excluded Information, example of locally selected wording
<section nullFlavor="MSK">
<templateId root="2.16.840.1.113883.10.20.22.2.7.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.7.1" extension="2014-06- 09"/>
<id root="4536582C-018F-11E6-9EF4-0050568B1D1B"/>
<code code="47519-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="History of procedures"/>
<title>Procedures - from Last 3 Months</title>
<text>
Information not provided due to security, privacy, or other reasons.
</text>
</section>
A study by the Association for Healthcare Documentation Integrity, presented at the HIMSS Health Story Project Roundtable on March 5, 2019, showed that consistent style in healthcare records enhances clear communication, boosts patient safety, and improves information exchange. Applying style standards to human-readable text can reduce physician burden, with a focus on improving the representation of temporal date/time information in the narrative text. While temporal details are vital for clinical understanding, excessive date/time precision can overwhelm or mislead. The Roundtable urged C-CDA creators to ensure that date/time information in the Narrative Block is relevant, pertinent, and appropriately styled to ease physician burden.
C-CDA Content Creators are encouraged to apply a consistent style to date/time information reported in the Narrative Blocks |
C-CDA Content Creators are encouraged to render date/time information using a level of precision that is relevant and pertinent to the intended purpose of the section within the context of the document. |
Time values are represented in ISO 8601 compliant form, which is traditional in HL7 and lacks dashes, colons, and a "T" between date and time. The syntax is "YYYYMMDDHHMMSS.UUUU[+|-ZZzz]," with the option to omit digits for less precision. Common forms are "YYYYMMDD" and "YYYYMMDDHHMM," but truncation on the right side can occur with other variants. The ".UUUU" part allows for precision down to tenths of a millisecond (e.g., ".001"). Timezone information is indicated using offsets from UTC, like -0500 for Eastern Standard Time (EST) and -0400 for Eastern Daylight Saving Time (EDT). UTC Time is represented as -0000. Without a timezone offset, assumptions about time should not be made unless there's a local exchange agreement. When timezone is NULL (unknown), it implies "local time," which is context-dependent and should not be converted to UTC. Specifying timezone for all TS values is important to avoid loss of precision during TS comparisons. In administrative data, values often lack a timezone. For instance, specifying a timezone for a date of birth in administrative data could alter the date when converted to other timezones. Therefore, administrative data typically has a NULL timezone (not applicable). See TS Point in Time in the StructureDefinition Publication of CDA V3 Data Types
When specifying dates and times, care should be taken to only capture data with as much precision as is known. The timestamp format allows for partial dates and partial times to be specified. For information see TS - Detailed Description from the CDA V3 Data Type Specification StructureDefinition publication.
The Entry Reference template represents the act of referencing another entry in the same CDA document instance. Its purpose is to remove the need to repeat the complete XML representation of the referred entry when relating one entry to another. This template can be used to reference many types of Act class derivations, such as encounters, observations, procedures etc., as it is often necessary when authoring CDA documents to repeatedly reference other Acts of these types. For example, in a Care Plan it is necessary to repeatedly relate Health Concerns, Goals, Activities and Outcomes.
The id is required and must be the same id as the entry/id it is referencing. The id cannot be a null value. Act/Code is set to nullFlavor=“NP” (Not Present). This means the value is not present in the message (in act/Code).
The <linkHtml> tag, is a generic referencing mechanism that can be used to reference identifiers that are internal
to a document. Note that security considerations need to be given to support for linking mechanisms. Not all
stylesheets enable the linking features of the <linkHtml> tag to be operationalized.
C-CDA Content Creators are encouraged to use id-based linking mechanisms within a C-CDA document. |
C-CDA Consumer are encouraged to support stylesheets that facilitate the use of internal linking mechanisms, because linking within a single file does not pose security risks, facilitates readability and. Improves user experience when viewing C-CDA documents. |
The base HL7 CDA standard supports four act classes that enable information in one document to reference information in an external document, external procedure, external observation, or external act.
C-CDA Content Creators are encouraged to explore the use of linking mechanisms to external C-CDA documents, observations, or acts, depending on business decisions and the assessment of risk associated with this functionality. |
C-CDA Content Consumers are encouraged to support stylesheets that support or prohibit the use of external linking mechanisms, depending on business decisions and the assessment of risk associated with this functionality. |
C-CDA entry templates include a negationInd attribute for Act classes (act, procedure, encounter, substanceAdministration, supply, and observation). When Act.negationInd is set to "true," it asserts that the Act as a whole is negated. Certain properties like Act.id, Act.moodCode, and participations remain unaffected and retain their original meaning.
A statement with negationInd still conveys information about the specific fact described by the Act. For example, a negated "finding of wheezing on July 1" means the author denies the presence of wheezing on that date, assuming the same responsibility and evidence requirement as for a non-negated statement.
In the case of observations, entry templates may specify whether the observation.negationInd attribute applies to the entire act or only to the observation.value element. CDA template definitions should explicitly describe this intention.
C-CDA Content Creators are strongly encouraged to follow template conformance and additional companion guidance regarding the use of negationInd when representing discrete data in C-CDA documents. |
C-CDA Content Consumers are strongly encouraged to follow template conformance and additional companion guidance regarding the use of negationInd when processing discrete data represented in C-CDA documents. |
This guidance is specific to individual entry templates and has been collected from various sources, including C-CDA implementer community feedback, published implementation guides, C-CDA Implementation-A-Thons,HL7's Cross Work Group work on mappings between C-CDA and FHIR, and efforts by the HL7 C-CDA Examples Task Force.
Each entry template described below includes its purpose, details about any associated state model, information on representing negated data, and special considerations from the C-CDA implementer community's experience.
The data classes listed provide guidance for relevant entry templates associated with each data class.
As demand increases for higher quality, more trusted clinical data in C-CDA documents, new efforts have emerged to clarify basic information requirements for representing data “provenance.” Provenance provides traceability of information and supports clinical information reconciliation and incorporation.
| Entry Template | Provenance Author Participation [author: identifier urn:oid:2.16.840.1.113883.10.20.22.5.6:2019-10-01 (open)] |
|---|---|
| Template | Provenance Author Participation |
| Purpose | This template provides a mechanism to record basic Provenance in an Author Participation. |
| ActStatus | Not applicable |
| Negation | Not applicable |
| Other Considerations | This template constrains the CDA Author Participation and is appropriate at the Header, Section, or Entry level. The conformance criteria specializes the C-CDA Author Participation (2.16.840.1.113883.10.20.22.4.119) but does not require generators to include this additional templateId. |
| Example | Example 33: Use of the Provenance Author Participation |
Table 36: Provenance Author Participation Template
Example 33: Use of the Provenance Author Participation
<section>
<!-- *** Allergies and Intolerances Section *** -->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<code code="48765-2" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>ALLERGIES AND ADVERSE REACTIONS</title>
<text ID="allergiesNoKnown">No Known Allergies</text>
<entry typeCode="DRIV">
<!-- Allergy Concern Act -->
<act classCode="A CT" moodCode="E VN">
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<templateId root="2.16.840.1.113883.10.20.22.4.30" extension="2015-08- 01"/>
<id root="36e3e930-7b14-11db-9fe1-0800200c9a66"/>
<!-- SDWG supports 48765-2 or CONC in the code element -->
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<text>
<reference value="#allergiesNoKnown"/>
</text>
<statusCode code="active"/>
<!--currently tracked concerns are active concerns-->
<effectiveTime>
<low value="20100903"/>
<!--show time when the concern first began being tracked-->
</effectiveTime>
<entryRelationship typeCode="SUBJ">
<!-- No Known Allergies -->
<!-- The negationInd = true negates the observation/value -->
<!-- The use of negationInd corresponds with the newer
Observation.valueNegationInd -->
<observation classCode="O BS" moodCode="E VN" negationInd="true">
<!-- allergy - intolerance observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<templateId root="2.16.840.1.113883.10.20.22.4.7" extension="2014-06- 09"/>
<id root="4adc1020-7b14-11db-9fe1-0800200c9a66"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<text>
<reference value="#allergiesNoKnown"/>
</text>
<statusCode code="completed"/>
<!-- N/A - author/time records when this assertion was made -->
<effectiveTime>
<low nullFlavor="NA"/>
</effectiveTime>
<value xsi:type="CD" code="419199007"
displayName="Allergy to substance (disorder)"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"/>
<author>
<!-- Provenance - Author Participation -->
<templateId root="2.16.840.1.113883.10.20.22.5.6" extension="2019-10- 01" />
<time value="201308011235-0800"/>
<assignedAuthor>
<!-- NPI of Author (example) -->
<id root="2.16.840.1.113883.4.6" extension="1234567"/>
<assignedPerson>
<name>
<given>Nurse</given>
<family>Nightingale</family>
<suffix>RN</suffix>
</name>
</assignedPerson>
<representedOrganization>
<!-- Tax Identifier of Organization is Unknown -->
<id root="2.16.840.1.113883.5.1008" nullFlavor="U NK"/>
<!-- NPI of Organization -->
<id root="2.16.840.1.113883.4.6" extension="1104145838"/>
<name>Good Health Hospital</name>
<telecom value="tel:+1(555)867-5309"/>
</representedOrganization>
</assignedAuthor>
</author>
<!-- In C-CDA R2.1 the participant is required. -->
<participant typeCode="C SM">
<participantRole classCode="MANU">
<playingEntity classCode="MMAT">
<code nullFlavor="NA"/>
</playingEntity>
</participantRole>
</participant>
</observation>
</entryRelationship>
</act>
</entry>
</section>
Systems can use the Provenance Author Participation to identify the author of content. This corresponds to a FHIR Provenance.agent.type="author" with a relevant Provenance.target.
When a system transforms a CDA entry using a Provenance - Author template assertion, the information from the template should go into the FHIR Provenance.agent and may also fill an appropriate Resource element.
If a CDA entry lacks an explicit Provenance-Author template assertion, it may not provide enough role specificity for populating a FHIR Provenance Resource with certainty.
Implementers will need to determine suitable mappings based on the specific situation.
The Section Time Range Observation defines a machine-readable business rule for restricting the time interval of information within a section. It's optional and can be applied in any section to avoid overwhelming or irrelevant content. This entry links to human-readable information that explains the applied business rules found in the section's narrative text.
| Entry Template | Section Time Range Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.201:2016-06-01 (open)] |
|---|---|
| Template | Section Time Range Observation |
| Purpose | This observation describes a date/time range applied by the document creator to limit the range of information contained in a section. |
| ActStatus | Section Time Range observation always has a statusCode of “completed”. This template does not include an effectiveTime element. See other considerations below. |
| Negation | Not specified. |
| Other Considerations | Narrative text linking applies for this entry. The human readable information describing the date/time range used to limit the information SHOULD be reported in the Narrative Block and SHOULD NOT be implied by the section.title only. The specified date/time range of the content limit is specified in the observation.value element. |
| Example | Example 34: Section Time Range Template Example |
Table 37: Section Time Range Observation Template
Example 34: Section Time Range Template Example
<section>
...
<title>Procedures</title>
<text>
<content ID="Proc_STR">Procedures performed between 08/15/2012 and 08/15/2015.</content>
...
</text>
<entry>
<observation classCode="O BS" moodCode="E VN">
<templateId root="2.16.840.1.113883.10.20.22.4.201" extension="2016-06- 01"/>
<code code=" 82607-3" codeSystem="2.16.840.1.113883.6.1"
displayName="Section Date and Time Range"/>
<text>
<reference value="#Proc_STR"/>
</text>
<statusCode code="completed"/>
<value xsi:type="IVL_TS">
<low value="20120815"/>
<high value="20150815"/>
</value>
</observation>
</entry>
</section>
The C-CDA document header contains roles filled by those involved in patient care. The roles are implicit, but the Care Teams Section and Care Team Organizer templates allow for explicit documentation of care team information. These templates offer more details about the type of care team and the roles of its members, providing greater expressiveness.
| Entry Template | Care Team Organizer [organizer: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.500:2019-07-01 (open)] |
|---|---|
| Template | Care Team Organizer |
| Purpose | This organizer template contains information about a single care team. The author of the organizer is the person who documented the care team information. The participants of the organizer are the care team lead(s) and the care team organization. The components of the organizer contain the following information: - The encounter that caused the care team to be formed - Narrative information about the care team - The care team members - Reasons for the care team - The care team type(s) - a care team can have multiple care team types |
| ActStatus | The actStatus of this entry is statically bound to ValueSet ActStatus urn:oid:2.16.840.1.113883.1.11.15933. Implementers need to be prepared to address the specified state model. Possible states include: active, completed, cancelled, held, suspended, new, normal, nullified, obsolete, and aborted. |
| Negation | Not explicitly specified. |
| Other Considerations | Implementers are encouraged to take guidance from the structure of this entry, even if only populating the Narrative Block of the Care Teams Section. Implementers should note the functionCode element vocabulary binding extends the set of concepts for a Care Team member’s role on the Care Team. A new value set called “Care Team Member Function” is grouped with the original value set called “ParticipationFunction.” In cases where a concept overlaps between these two value sets, implementers should use the concept from the ParticipationFunction value set. |
| Example | Example 35: Care Teams Section with Care Team Member Organizer for discrete data representation. |
Table 38: Care Team Organizer Template
 |
Logical design for Care Team Organizer Template
Example 35: Care Teams Section with Care Team Member Organizer for discrete data representation.
<section>
<!-- Care Teams Section Template ID and extension-->
<templateId root="2.16.840.1.113883.10.20.22.2.500" extension="2019-07- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.2.500" extension="2022-06- 01"/>
<code code="85847-2" codeSystem="2.16.840.1.113883.6.1"/>
<title>Care Teams</title>
<text>
<list>
<item>
<content ID= "CareTeamName1">Inpatient Diabetes Care Team</content>
(<content>Active</content>)
<table>
<thead>
<tr>
<th>Member</th>
<th>Role on Team</th>
<th>Status</th>
<th>Date</th>
</tr>
</thead>
<tbody>
<tr>
<td>Dr. Henry Seven </td>
<td ID="CT1_M01">PCP</td>
<td>(Active)</td>
<td>10/18/2019</td>
</tr>
</tbody>
</table>
</item>
</list>
</text>
<!--Care Team Organizer-->
<entry>
<organizer classCode="CLUSTER" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.500"
extension="2019-07- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.500"
extension="2022-06- 01"/>
<!--NEW Care Team Organizer Entry Template ID and extension-->
<id root="1.1.1.1.1.1"/>
<code code="86744-0" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Care Team">
<originalText>
<reference value="#CareTeamName1"/>
</originalText>
</code>
<!--Care Team Status -
https://vsac.nlm.nih.gov/valueset/2.16.840.1.113883.1.11.15933/expansion-->
<statusCode code="active"/>
<effectiveTime>
<low value="201810081426-0500"/>
</effectiveTime>
<!-- This participant is the Care Team Lead (1..1)-->
<!-- Care Team Lead is one of the contained care team members in the list of care
team members-->
<participant typeCode="PPRF">
<participantRole>
<!--<This id matches at least one of the member's id in the Care Team
Member act template-->
<id root="1.5.5.5.5.5.5"/>
</participantRole>
</participant>
<!-- #1 Care Team Member Act - This component is a care team member who is a
provider -->
<component>
<act classCode="PCPR" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.500.1"
extension="2019-07- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.500.1"
extension="2022-06- 01"/>
<id root="1.5.5.5.5.5.5"/>
<code code="85847-2" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Care Team Information"/>
<!--Care Team Member Status -
https://vsac.nlm.nih.gov/valueset/2.16.840.1.113883.1.11.15933/expansion-->
<statusCode code="active"/>
<effectiveTime xsi:type="IVL_TS">
<low value="201810081426-0500"/>
</effectiveTime>
<!--Attributes about the provider member - name-->
<performer typeCode="PRF">
<functionCode
xmlns="urn:hl7-org:sdtc" code="PCP"
displayName="primary care physician"
codeSystem="2.16.840.1.113883.5.88"
codeSystemName="ParticipationFunction">
<originalText
xmlns="urn:hl7-org:v3">
<reference value="#CT1_M01"/>
</originalText>
</functionCode>
<!-- A care team member role -->
<assignedEntity>
<id root="B00B14E8-CDE4-48EA-8A09-01BC4945122A"
extension="1"/>
<id root="1.5.5.5.5.5.5"/>
<assignedPerson>
<name>
<given>John</given>
<given>D</given>
<family>Smith</family>,
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedEntity>
</performer>
</act>
</component>
</organizer>
</entry>
</section>
The Encounters Section lists past and ongoing patient encounters, including the one that led to the document's creation. Future appointments and requested encounters should be mentioned in the Plan of Treatment Section.
When the document pertains to a single encounter, the Encounter section needs to contain information about that encounter, but can possibly also contain additional encounters. |
The Encounter Activity entry with a matching id in the encompassingEncounter header represents the primary encounter.
Encounter Diagnosis is always shown as an entryRelationship to an Encounter Activity, even for single encounters. Historical encounters are each documented as an Encounter Activity, with information recorded through entryRelationships within the corresponding Encounter Activity. Additional details, like free-text notes, can be conveyed through extra entryRelationships within the associated Encounter Activity.
To add a note to the Encounter, use the Note Activity entry, and link it with an entryRelationship to this new Note Activity entry template.
| Entry Template | Encounter Activity [encounter: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.49:2015-08-01 (open)] |
|---|---|
| Template | Encounter Activity |
| Purpose | This clinical statement describes an interaction between a patient and clinician. Interactions may include in-person encounters, telephone conversations, and email exchanges. |
| ActStatus | No constraint specified. |
| Negation | No constraint specified. |
| Other Considerations | This template may leverage the sdtc:dischargeDispositionCode extension, which records patient discharge disposition information. It may also include data on involved practitioners, encounter locations, encounter reasons, and multiple encounter diagnoses. |
| Reference | Visit HL7 CDA Example Search |
| Example | How to represent Outpatient Encounter with Diagnoses |
Table 39: Encounter Activity Template
| Entry Template | Encounter Diagnosis [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.80:2015-08-01 (open)] |
|---|---|
| Template | Encounter Diagnosis |
| Purpose | This template wraps relevant problems or diagnoses at the close of a visit or that need to be followed after the visit. |
| ActStatus | Fixed to 'completed'. |
| Negation | No constraint specified. |
| Other Considerations | This template requires at least one contained Problem Observation template. |
| Reference | Visit HL7 CDA Example Search |
| Example | How to represent Outpatient Encounter with Diagnoses |
Table 40: Encounter Diagnosis Template
| Entry Template | Hospital Admission Diagnosis [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.34:2015-08-01 (open)] |
|---|---|
| Template | Hospital Admission Diagnosis |
| Purpose | This template represents problems or diagnoses identified by the clinician at the time of the patient’s admission. |
| ActStatus | No constraint specified. |
| Negation | Not explicitly specified. |
| Other Considerations | This Hospital Admission Diagnosis act can contain multiple Problem Observations to represent multiple diagnoses during hospital admission. The only distinction from a Hospital Discharge Diagnosis is the LOINC code in the Act.code element, which is 46241-6 for Hospital Admission Diagnosis. (Hospital admission diagnosis Narrative - Reported), while Hospital Discharge Diagnosis uses LOINC code 11535-2 (Hospital discharge Dx Narrative) |
| Example | Example 36: Hospital Admission Diagnosis |
Table 41: Hospital Admission Diagnosis Template
Example 36: Hospital Admission Diagnosis
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.34" extension="2015-08-01" />
<id root="5a784260-6856-4f38-9638-80c751aff2fb" />
<code code="46241-6" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Hospital Admission Diagnosis" />
<statusCode code="active" />
<effectiveTime>
<low value="20090303" />
</effectiveTime>
<entryRelationship typeCode="SUBJ" inversionInd="false">
<observation classCode="OBS" moodCode="EVN">
| Entry Template | Hospital Discharge Diagnosis [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.33:2015-08-01 (open)] |
|---|---|
| Template | Hospital Discharge Diagnosis |
| Purpose | This template represents problems or diagnoses present at the time of discharge which occurred during the hospitalization or need to be monitored after hospitalization. |
| ActStatus | No constraint specified. |
| Negation | Not explicitly specified. |
| Other Considerations | This template requires at least one contained Problem Observation template. The primary difference between a Discharge Diagnosis and a Hospital Discharge Diagnosis is the LOINC code used in the Act.code element of the act wrapper. Encounter Diagnosis uses LOINC code 29308-4 (Diagnosis), while Hospital Discharge Diagnosis uses LOINC code 11535-2 (Hospital discharge Dx Narrative) |
| Reference | Hospital Discharge Diagnosis |
| Example | How to reference Hospital Discharge Encounter with Billable Diagnoses |
Table 42: Hospital Discharge Diagnosis Template
Fulfilled Orders: The inFulfillmentOf/order in the document header records fulfilled orders within the Encompassing Encounter or Service Event. For instance, when a provider orders an X-Ray, and it's performed, the X-Ray order identifier goes in inFulfillmentOf/order, while the performed X-Ray procedure is documented in documentationOf/ServiceEvent. In the document body, templates are used to represent the service activities fulfilling the order, such as tests, procedures, substance administrations, supplies, encounters, or other acts.
C-CDA Content Creators are strongly encouraged to represent completed orders in the inFulfillmentOf area of the header for orders completed in the context established for the document. |
C-CDA Content Consumers are strongly encouraged to render information about completed orders documented in the inFulfillmentOf area of the header for orders completed in the context established for the document. For example, a consumer might opt to render any inFulfillmentOf/order/id elements where @displayable="true", the inFulfillmentOf/order/code/originalText or @displayName, or both. |
Entry templates defined to represent performed service acts are Intervention Act, Procedure Activity Procedure, Immunization Activity, Encounter Activity, Medication Activity, Non-Medicinal Supply Activity, etc. Templates types are discussed in the context of the data class used to categorize the type of service act.
Entry templates defined to represent ordered service acts such as Planned Encounter, Planned Medication Activity, Planned Procedure, Planned Supply, etc.
Templates of these types are discussed in the context of the data class used to categorize the type of service activity. The key distinction for representing a placed order is to utilize the moodCode attribute with a value of RQO. RQO in comes from the HL7 ActMood code system and conceptually means “requested”. It is used to represent an ordered service activity.
Problem information encompasses health concerns, problem concerns, and problem observations, including statements about no known allergies.
A patient's problem is represented using templates designed for health concerns, which describe health-related matters of interest, importance, or worry, derived from various EHR sources. Problem concerns are a specific subset that typically appear on a "Problem List" and are of broad interest to the care team. Other health concerns, not typically considered problem concerns, address specific issues like transportation difficulties or underinsurance. Risk concerns, like "Risk of Hyperkalemia" for a patient taking an ACE-inhibitor medication, are also identified.
Currently, the Problem Section uses the Problem Concern template for concerns on the patient's problem list. The Health Concerns section employs Health Concern and Risk Concern templates for broader concerns and risks not typically on the problem list.
Implementers face challenges when source information doesn't align with the designed clinical statement patterns, especially regarding the concern pattern because many EHR systems organize source problem data differently in the patient's record.
| Entry Template | Problem Concern Act [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.3:2015-08-01 (open)] |
|---|---|
| Template | Problem Concern Act |
| Purpose | This template reflects an ongoing concern on behalf of the provider who is managing the patient’s condition. |
| ActStatus | The statusCode of a concern is "active" as long as it's of interest to the provider, regardless of whether the condition is resolved. It becomes "completed" only when the condition is no longer a concern. The effectiveTime reflects when the condition was concerning, which may differ from the condition's actual timeline. The statusCode of the Problem Concern Act indicates the concern's status, while the effectiveTime of the nested Problem Observation specifies when the patient experienced the problem. The effectiveTime/low of the Problem Concern Act marks when the concern became active, and the effectiveTime/high indicates when it was completed, i.e., when there's no longer a need to track the condition. |
| Negation | Not explicitly specified. |
| Other Considerations | A Problem Concern Act can contain one or more Problem Observations (templateId 2.16.840.1.113883.10.20.22.4.4). In practice, most EHRs do not support this template design. See best practice guidance below for implementers who do not support problem concern tracking at this time. Visit HL7 CDA Example Search for representing the expression “No Known Problems” |
| Reference | Visit HL7 CDA Example Search |
| Example | Example 37: Problem Concern containing a Problem Observation |
Table 43: Problem Concern Template
The following is guidance with respect to the relationship between the Problem Concern Act and the Problem Observation.
C-CDA Content Creators who do not natively support organizing a patient’s problem observations into collections associated with a tracked concern are encouraged to use the following guidance: 1. Populate the Act.statusCode of the Problem Concern Act to reflect the status of the clinical statement about the problem stored within the source system. 2. Use nullFlavor=”NI” for the effectiveTime of the outer concern act wrapper. 3. Do not populate the author participation template associated with the outer concern act wrapper. 4. Include a single Problem Observation within the act wrapper. A future release of C-CDA will only allow the following within the act wrapper: only 1 entryRelationship of type REFR or COMP (1..1 entryRelationship of @typeCode=REFR OR @typeCode=COMP), and any number of supporting entryRelationships (0..* entryRelationship of @typeCode=SPRT) 5. Populate the effectiveTime of the Problem Observation with the clinically relevant time period associated with problem. 6. Include the author participation template associated with the Problem Observation to record the person who documented the problem. 7. Use the performer associated with the Problem Observation to record the person who made the diagnosis or observed the problem if that person is not also the author. 8. Use the Problem Status Observation template to record the clinical status assigned to the problem. Note that clinical status is a judgement assigned by the performer of the observation. |
Example 37: Problem Concern containing a Problem Observation
C-CDA Examples Task Force Problem Section entry examples
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.5.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.5.1" extension="2015-08- 01"/>
<id root="093A5380-00CE-11E6-B4C5-0050568B000B"/>
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="PROBLEM LIST"/>
<title>Problem List</title>
<text>
<table width="100%">
<thead>
<tr>
<th>Problem Concern Information | Tracked By</th>
<th>Condition(s)</th>
</tr>
</thead>
<tbody>
<tr styleCode="normRow">
<td ID="ProblemConcern_1"><content>Active Problem</content> | <content/></td>
<td>
<list>
<item>
<table>
<thead>
<tr>
<th>Problem Type | Problem<br/></th>
<th>Time Frame</th>
<th>Clinical Status</th>
<th>Documented By</th>
</tr>
</thead>
<tbody>
<tr ID="ProblemObs1">
<td>
<content ID="ProblemObs_1_PT1">Problem</content> | <content
styleCode="Bold" ID="ProblemObs_1_P1">Osteoarthritis</content>
</td>
<td><content>(09/09/2014 - )</content></td>
<td><content ID="ProblemObs_1_PS1">Active</content></td>
<td><content>W.Colon</content> <content>(06/18/2015)</content></td>
</tr>
</tbody>
</table>
</item>
</list>
</td>
</tr>
</tbody>
</table>
</text>
<entry>
<act classCode="ACT" moodCode="EVN">
<!-- Problem Concern -->
<templateId root="2.16.840.1.113883.10.20.22.4.3"/>
<templateId root="2.16.840.1.113883.10.20.22.4.3" extension="2015-08- 01"/>
<id extension="68993" root="1.2.840.114350.1.13.6289.1.7.2.768076"/>
<id root="093A5380-00CE-11E6-B4C5-0050568B000B" extension="1"/>
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"
codeSystemName="HL7ActClass" displayName="Concern"></code>
<!-- This shows what to do when the source system does not track problem concerns. -->
<text>
<reference value="#ProblemConcern_1"></reference>
</text>
<statusCode code="active"/>
<effectiveTime nullFlavor="NI"/>
<!-- A System that supports Concern tracking MAY include the author(s) of the Concern
in addition to
author(s) for the contained Problem Observation(s). -->
<entryRelationship inversionInd="false" typeCode="SUBJ">
<!-- Problem Observation -->
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2022-06-01"/>
<id extension="68993" root="1.2.840.114350.1.13.6289.1.7.2.768076"/>
<id root="093A5380-00CE-11E6-B4C5-0050568B000B" extension="1.1"/>
<code code="55607006" codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"
displayName="Problem">
<originalText>
<reference value="#ProblemObs_1_PT1"/>
</originalText>
<translation code="75326-9" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Problem"/>
</code>
<text>
<reference value="#ProblemObs1"/>
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="20140909"/>
</effectiveTime>
<value code="396275006" codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"
displayName="Osteoarthritis"
xsi:type="CD"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance">
<originalText>
<reference value="#ProblemObs_1_P1"/>
</originalText>
<translation code="715.90" codeSystem="2.16.840.1.113883.6.103" codeSystemName="ICD-
9CM" displayName="Osteoarthritis"/>
<translation code="M19.90" codeSystem="2.16.840.1.113883.6.90" codeSystemName="ICD-10-
CM" displayName="Osteoarthritis"/>
</value>
<author>
<time value="20150618"/>
<assignedAuthor>
<id nullFlavor="UNK"/>
<addr>
<streetAddressLine>8333 Clairemont Mesa Blvd.</streetAddressLine>
<city>Bel Air</city>
<state>MD</state>
<postalCode>21014</postalCode>
</addr>
<telecom use="WP" value="tel:+1-410-555-5544"/>
<assignedPerson>
<name>
<given>Wilma</given>
<family>Colon</family>
</name>
</assignedPerson>
<representedOrganization>
<name>Good Health Hospital</name>
<telecom use="WP" value="tel:+1-410-555-5544"/>
<addr>
<streetAddressLine>8333 Clairemont Mesa Blvd.</streetAddressLine>
<city>Bel Air</city>
<state>MD</state>
<postalCode>21014</postalCode>
</addr>
</representedOrganization>
</assignedAuthor>
</author>
<entryRelationship typeCode="REFR">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.6"/>
<code code="33999-4" codeSystem="2.16.840.1.113883.6.1" displayName="Status"/>
<text>
<reference value="#ProblemObs_1_PS1"></reference>
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="20140909"/>
</effectiveTime>
<value code="55561003" codeSystem="2.16.840.1.113883.6.96" displayName="Active"
xsi:type="CD"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"/>
</observation>
</entryRelationship>
</observation>
</entryRelationship>
</act>
</entry>
</section>
| Entry Template | Problem Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.4:2022-06-01 (open)] |
|---|---|
| Template | Problem Observation |
| Purpose | This template reflects a discrete observation about a patient's problem. |
| ActStatus | This template always has a statusCode of “completed”. The effectiveTime, also referred to as the “clinically relevant time” is the time at which the observation holds true for the patient. For a provider seeing a patient in the clinic today, observing a history of heart attack that occurred five years ago, the effectiveTime is five years ago. The effectiveTime of the Problem Observation is the definitive indication of when the problem occurred. If the problem is known to be resolved, then an effectiveTime/high would be present. If the date of resolution is not known, then effectiveTime/high will be present with a nullFlavor of "UNK". |
| Negation | In this template, the negationInd attribute is used to indicate the absence of the condition in observation/value (Observation.ValueNegationInd). |
| Other Considerations | The optional Problem Status Observation template (identifier: urn:oid:2.16.840.1.113883.10.20.22.4.6) represents a clinical judgement of the status of the problem. negationInd of "true" coupled with an observation/value of SNOMED code 55607006 "Problem" indicates that the patient has no known conditions. |
| Example | Example 37: Problem Concern containing a Problem Observation |
| Example | Example 38: No Known Problems |
Table 44: Problem Observation Template
Example 38: No Known Problems
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.5.1"/>
<!-- Problem Section -->
<templateId root="2.16.840.1.113883.10.20.22.2.5.1" extension="2015-08- 01"/>
<!-- Problem Section -->
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Problem List"/>
<title>PROBLEMS</title>
<text ID="Concern_1"> Problem Concern:<br/> Concern Tracker Start Date: 06/07/2013
16:05:06<br/>
Concern Tracker End Date: <br/> Concern Status: Active<br/>
<content ID="problems1">No known <content ID="problemType1">problems.</content></content>
</text>
<entry typeCode="DRIV">
<!-- Problem Concern Act -->
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.3"/>
<templateId root="2.16.840.1.113883.10.20.22.4.3" extension="2015-08- 01"/>
<id root="36e3e930-7b14-11db-9fe1-0800200c9a66"/>
<!-- SDWG supports 48765-2 or CONC in the code element -->
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<text>
<reference value="#Concern_1"/>
</text>
<statusCode code="active"/>
<!-- So long as the underlying condition is of concern to the provider (i.e., as long
as the condition,
whether active or resolved, is of ongoing concern and interest to the
provider), the statusCode is “active”.
Only when the underlying condition is no longer of concern is the statusCode
set to “completed”. -->
<effectiveTime>
<low value="20130607160506"/>
<!-- The effectiveTime reflects the time that the underlying condition was felt
to be a concern;
it may or may not correspond to the effectiveTime of the condition (e.g.,
even five years later, the clinician may remain concerned about a prior heart attack).-->
</effectiveTime>
<!-- status is active so high is not applicable. If high is present it should have
nullFlavor of NA-->
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<time value="20130607160506"/>
<assignedAuthor>
<id extension="66666" root="2.16.840.1.113883.4.6"/>
<code code="207RC0000X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC" displayName="Cardiovascular Disease"/>
<addr>
<streetAddressLine>6666 StreetName St.</streetAddressLine>
<city>Silver Spring</city>
<state>MD</state>
<postalCode>20901</postalCode>
<country>US</country>
</addr>
<telecom value="tel:+1(301)666-6666" use="WP"/>
<assignedPerson>
<name>
<given>Heartly</given>
<family>Sixer</family>
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedAuthor>
</author>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN" negationInd="true">
<!-- The negationInd is used to indicate the absence of the condition in
observation/value (Observation.ValueNegationInd).
A negationInd of "true" coupled with an observation/value of SNOMED
code 55607006 "Problem"
indicates that the patient has no known conditions. -->
<!-- problem observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2022-06- 01"/>
<id root="4adc1021-7b14-11db-9fe1-0800200c9a67"/>
<code code="64572001" displayName="Disease"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT">
<translation code="75323-6" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Condition"/>
</code>
<text>
<reference value="#problems1"/>
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="20130607160506"/>
</effectiveTime>
<!-- The time when this was clinically relevant ie True for the patient. -->
<!-- As a minimum time interval over which this is true, populate the
effectiveTime/low with the current time. -->
<!-- It would be equally valid to have a longer range of time over which this
statement was represented as being true. -->
<!-- As a maximum, you would never indicate an effectiveTime/high that was
greater than the current point in time. -->
<!-- This idea assumes that the value element could come from the Problem
value set, or-->
<!-- when negationInd was true, is could also come from the ProblemType value
set (and code would be ASSERTION). -->
<value xsi:type="CD" code="55607006" displayName="Problem"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT">
<originalText>
<reference value="#problemType1"/>
</originalText>
</value>
</observation>
</entryRelationship>
</act>
</entry>
</section>
A health concern signifies interest or worry about a health state, possibly requiring attention. It's a health-related matter of importance to the patient, family, or healthcare provider, derived from various EHR sources. Problem Concerns are a subset of Health Concerns, specifically those on the patient's "Problem List."
A Health Concern Act tracks suboptimal physical or psychological situations drawing the patient to healthcare. If the underlying condition remains of concern, the statusCode is "active"; it switches to "completed" when it's no longer a concern. The effectiveTime marks when the condition became concerning, not necessarily corresponding to the condition's timeline. Health concerns guide interventions to achieve care goals.
A Risk Concern Act represents potential health issues needing intervention. It's derived similarly to Health Concerns but carries different semantics with Act.code. The templates are otherwise identical, following implementer best practices for Health Concerns.
In summary, Health Concerns express health-related worries, Problem Concerns are those on the Problem List, and Risk Concerns address potential issues requiring attention, all tracked with similar templates.
| Entry Template | Health Concern [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.132:2022-06-01 (open)] |
|---|---|
| Template | Health Concern Act |
| Purpose | This template represents a single health concern which may be derived from a variety of sources within an EHR (such as Problem List, Family History, Social History, Social Worker Note, etc.). A Health Concern is used to track non-optimal physical or psychological situations drawing the patient to the healthcare system. These may be from the perspective of the care team or from the perspective of the patient. |
| ActStatus | When the underlying condition is of concern (i.e., as long as the condition, whether active or resolved, is of ongoing concern and interest), the statusCode is “active”. Only when the underlying condition is no longer of concern is the statusCode set to “completed”. The effectiveTime reflects the time that the underlying condition was felt to be a concern; it may or may not correspond to the effectiveTime of the condition (e.g., even five years later, a prior heart attack may remain a concern). |
| Negation | Not explicitly specified. |
| Other Considerations | In its simplest form, a Health Concern template can be used to wrap a Problem Observation template. Also, best practice guidance associated with the Problem Concern Act template can be applied similarly to the Health Concern template. Examples of a Health Concern that might not be considered a Problem Concern include: "Risk of Hyperkalemia” for a patient taking an ACE-inhibitor medication, “Transportation difficulties” for a patient who doesn’t drive and has trouble getting to appointments, or “Under-insured” for a patient who doesn’t have sufficient insurance to cover their medical needs such as medications. Problem Concerns are a subset of Health Concerns. Problem Concerns are problems and diagnoses typically found on a classic “Problem List”, such as “Diabetes Mellitus” or “Family History of Melanoma” or “Multi-vessel Coronary Artery Disease”. |
| Example | Example 39: Health Concern narrative entry |
Table 45: Health Concern Template
Example 39: Health Concern narrative entry
<component>
<section>
<!-- Health Concerns Section -->
<templateId root="2.16.840.1.113883.10.20.22.2.58" extension="2015-08- 01"/>
<code code="75310-3" displayName="Health Concerns Document"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Health Concerns</title>
<text><paragraph ID="Concern">On March 1, 2014, the patient expressed concern about
spreading their Community Acquired Pneumonia.</paragraph></text>
<entry>
<!-- Health Concern Act -->
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.132" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.132" extension="2022-06-01"/>
<id nullFlavor="UNK"/>
<!-- Fixed act/code in C-CDA -->
<code code="75310-3" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Health Concern"/>
<text><reference value="#Concern"></reference></text>
<statusCode code="active"/>
</act>
</entry>
</section>
</component>
Allergy information includes medication and non-medication allergy concerns and observations including statements about no known allergies.
The Allergy Concern template groups multiple allergy intolerance observations into one concern. It represents patient allergies or intolerances that may need attention, intervention, or management and is typically used for items on the patient's allergy list. The Allergy Concern Act template serves as a wrapper for the underlying Allergy - Intolerance Observations.
| Entry Template | Allergy Concern Act [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.30:2015-08-01 (open)] |
|---|---|
| Template | Allergy Concern Act |
| Purpose | This template reflects an ongoing concern on behalf of the provider who is managing the patient’s care. |
| ActStatus | So long as the underlying allergy or intolerance is of ongoing concern and interest to the provider, the statusCode of the concern is “active”. Only when the underlying allergy or intolerance is no longer of concern is the statusCode of the Allergy Concern Act set to “completed.” The effectiveTime reflects the time that the underlying allergy or intolerance was felt to be a concern; it may or may not correspond to the effectiveTime of the observation associated with the actual allergic reaction experienced by the patient. The statusCode of the Allergy Concern Act is the status of the concern, whereas the effectiveTime of the nested Allergy - Intolerance Observation tells when the allergy or intolerance was experienced by the patient. The effectiveTime/low of the Allergy Concern Act asserts when the concern became active. The effectiveTime/high asserts when the concern was completed (e.g., when the clinician deemed there is no longer any need to track the underlying allergy or intolerance). |
| Negation | Not explicitly specified. |
| Other Considerations | An Allergy Concern Act can contain one or more Allergy - Intolerance Observations (templateId .16.840.1.113883.10.20.22.4.7). In practice, most EHRs do not support this template design. See best practice guidance below for implementers who do not support allergy concern tracking at this time. Visit HL7 CDA Example Search for representing the expression “No Known Allergies”, “No Known Medication Allergies”. |
| Example | Example 40: Allergy concern for food allergy to eggs |
| Example | Example 41: No known allergies |
| Example | Visit HL7 CDA Example Search for additional examples showing allergies to specific medication. |
Table 46: Allergy Concern Template
This guidance for allergy concerns is needed because mismatches between clinical statement patterns and source information pose challenges for implementers. Many EHR systems don't organize allergy data in this manner, creating implementation difficulties.
C-CDA Content Creators who do not natively support organizing a patient’s allergy/intolerance observations into collections associated with a tracked concern are encouraged to use the following guidance: 1. Populate the Act.statusCode of the Allergy Concern Act to reflect the status of the clinical statement about the allergy/intolerance stored within the source system. 2. Use nullFlavor=”NI” for the effectiveTime of the outer concern act wrapper. 3. Do not populate the author participation template associated with the outer concern act wrapper. 4. Include a single Allergy-Intolerance Observation within the act wrapper. A future release of C-CDA will only allow the following within the act wrapper: only 1 entryRelationship of type REFR or COMP (1..1 entryRelationship of @typeCode=REFR OR @typeCode=COMP), and any number of supporting entryRelationships (0..* entryRelationship of @typeCode=SPRT) 5. Populate the effectiveTime of the Problem Observation with the clinically relevant time period associated with allergy/intolerance. 6. Include the author participation template associated with the Allergy-Intolerance Observation to record the person who documented the problem. 7. Use the performer associated with the Allergy-Intolerance Observation to record the person who made the diagnosis or observed the allergy/intolerance. 8. Use the Allergy Status Observation template to record the clinical status assigned to the problem. Note that clinical status is a judgement assigned by the performer of the observation. |
Example 40: Allergy concern for food allergy to eggs Please see the Example Task Forces Allergy Examples
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<id root="0937FF9A-00CE-11E6-B4C5-0050568B000B"/>
<code code="48765-2" displayName="Allergies &or adverse reactions doc"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>ALLERGIES</title>
<text>
<table width="100%">
<thead>
<tr>
<th>Concern Information | Tracked By</th>
<th>Allergy - Intolerance Information</th>
</tr>
</thead>
<tbody>
<tr ID="AllergyConcern_1" styleCode="normRow">
<td>
<content>Active (01/04/2014 - )</content> | <content>(01/04/2019)
H.Seven</content>
</td>
<td>
<list>
<item>
<table>
<thead>
<tr>
<th>Allergy Type | Allergen<br/></th>
<th>Criticality</th>
<th>Reaction | Severity<br/></th>
<th>Time Frame</th>
<th>Clinical Status</th>
<th>Documented By</th>
</tr>
</thead>
<tbody>
<tr ID="AllergyConcern_1_Allergn_1">
<td><content>Food allergy</content> | <content
styleCode="Bold" ID="AllergyConcern_1_Allergen_1"
>Egg</content>
</td>
<td>
<content ID="AllergyConcern_1_Criticality_1">High
Criticality</content>
</td>
<td>
<content ID="AllergyConcern_1_Reaction_1"
>Hives</content> | <content
ID="AllergyConcern_1_ReacSeverity_1"
>Moderate</content>
</td>
<td>
<content>(1998)</content>
</td>
<td>
<content>Active</content>
</td>
<td>
<content>H.Provider</content>
<content>(01/04/2014)</content>
</td>
</tr>
</tbody>
</table>
</item>
</list>
</td>
</tr>
</tbody>
</table>
</text>
<entry typeCode="DRIV">
<act classCode="A CT" moodCode="E VN">
<!-- ** Allergy Concern act ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<templateId root="2.16.840.1.113883.10.20.22.4.30" extension="2015-08- 01"/>
<id root="0fffb34f-c1e0-47c2-92af-c414a3ff21ec"/>
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<text>
<reference value="#AllergyConcern_1"/>
</text>
<statusCode code="active"/>
<!-- This is the time stamp for when the allergy was first documented as a concern-->
<effectiveTime>
<low value="20140104123506- 08 00"/>
</effectiveTime>
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<time val8e="20140104"/>
<assignedAuthor>
<id extension="99999999" root="2.16.840.1.113883.4.6"/>
<code code="207Q00000X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="Health
Care Provider Taxonomy" displayName="Family medicnne"/>
<telecom use="WP" value="tel:555-555-1002"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
<entryRelationship typeCode="SUBJ">
<observation classCode="O BS" moodCode="E VN">
<!-- allergy observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<templateId root="2.16.840.1.113883.10.20.22.4.7" extension="2014-06- 09"/>
<id root="0fffb34f-c1e0-47c2-92af-c414a3ff21ec"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<text>
<reference value="#AllergyConcern_1_Allergy_1"/>
</text>
<statusCode code="completed"/>
<!-- This is the time stamp for the clinical onset of the allergy. -->
<!-- Just the year is shown since a specific month and date was not reported -->
<effectiveTime>
<low value="1998"/>
</effectiveTime>
<!-- This specifies that the allergy is to a food in contrast to other allergies
(drug) -->
<value xsi:type="CD" code="414285001" displayName="Food allergy (disordor)"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"/>
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<time value="20140104"/>
<assignedAuthor>
<id extension="99999999" root="2.16.840.1.113883.4.6"/>
<code code="207Q00000X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="Health Care Provider Taxonomy"
displayName="Family Medicine"/>
<telecom use="WP" value="tel:555-555-1002"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
<!-- In C-CDA R2.1 the participant is required. -->
<participant typeCode="C SM">
<participantRole classCode="MANU">
<playingEntity classCode="MMAT">
<!-- The agent responsible for an allergy or adverse reaction is not always a
manufactured material (for example, food allergies),
nor is it necessarily consumed. The following constraints reflect limitations in the base CDA R2
specification,
and should be used to represent any type of responsible agent, i.e., use playingEntity
classCode“= "MMAT" for all agents,
manufactured or not. -->
<!--
... the expectation for use is that the chosen concept identifier for a substance should be
appropriately specific and drawn from the available code systems in the following priority order:
NDFRT, then RXNORM, then SNOMEDCT. UNII was in an earlier version of this grouping value set but
has been removed due to lack of industry use
-->
<code code="102263004" displayName="Eggs (edible)"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT">
<originalText>
<reference value="#AllergyConcern_1_Allergen_1"/>
</originalText>
</code>
</playingEntity>
</participantRole>
</participant>
<entryRelationship typeCode="SUBJ" inversionInd="true">
<observation classCode="O BS" moodCode="E VN">
<templateId root="2.16.840.1.113883.10.20.22.4.145"/>
<code code="82606-5" codeSystem="2.16.840.1.113883.6.1"
displayName="Criticality"/>
<text>
<reference value="#AllergyConcern_1_Criticality_1"/>
</text>
<statusCode code="completed"/>
<value xsi:type="CD" code="CRITH" displayName="High Criticality"
codeSystem="2.16.840.1.113883.5.1.63"
codeSystemName="HL7ObservationValue"/>
</observation>
</entryRelationship>
<entryRelationship typeCode="MFST" inversionInd="true">
<observation classCode="O BS" moodCode="E VN"> —-- Reaction Observation
template --> <templateId root="2.16.840.1.113883.10.20.22.4.9"/>
<templateId root="2.16.840.1.113883.10.20.22.4.9"
extension="2014-06- 09"/>
<id root="d89ce431-e0f1-4f8d-a81f-489b6ed91f09"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<text>
<reference value="#AllergyConcern_1_Reaction_1"/>
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="1998"/>
<high nullFlavor="U NK"/>
</effectiveTime>
<value xsi:type="CD" code="247472004"
codeSystem="2.16.840.1.113883.6.96" codeSystemNaee="SNOMED CT"
displayName="Hives"/>
<entryRelationship typeCode="SUBJ" inversionInd="true">
<observation classCode="O BS" moodCode="E VN"> —-- Severity
Observation template --> <templateId
root="2.16.840.1.113883.10.20.22.4.8"/>
<templateId root="2.16.840.1.113883.10.20.22.4.8"
extension="2014-06- 09"/>
<code code="S EV" codeSystem="2.16.840.1.113883.5.4"
codeSystemName="ActCode"/>
<text>
<reference value="#AllergyConcern_1_ReacSeverity_1"/>
</text>
<statusCode code="completed"/>
<value xsi:type="CD" code="6736007"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT" displayName="moderate"/>
</observation>
</entryRelationship>
</observation>
</entryRelationship>
</observation>
</entryRelationship>
</act>
</entry>
</section>
Example 41: No known allergies
<section>
<!-- *** Allergies and Intolerances Section *** -->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<code code="4876 5 - 2" codeSystem="2.16.840.1.113883. 6 .1" codeSystemName="LOINC"/>
<title>ALLERGIES AND ADVERSE REACTIONS</title>
<text ID="allergiesNoKnown">No Known Allergies</text>
<entry typeCode="DRIV">
<!-- Allergy Concern Act -->
<act classCode="A CT" moodCode="E VN">
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<templateId root="2.16.840.1.113883.10.20.22.4.30" extension="2015-08- 01"/>
<id root="36e3e930-7b14-11db-9fe1-0800200c9a66"/>
<!-- SDWG supports 48765-2 or CONC in the code element -->
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<text>
<reference value="#allergiesNoKnown"/>
</text>
<statusCode code="active"/>
<!--currently tracked concerns are active concerns-->
<effectiveTime>
<low value="20100103"/>
<!--show time when the concern first began being tracked-->
</effectiveTime>
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<time value="20100103"/>
<assignedAuthor>
<id extension="99999999" root="2.16.840.1.113883.4.6"/>
<code code="207Q00000X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="Health Care Provider Taxonomy" displayName="Family Medicine"/>
<telecom use="WP" value="tel:555-555-1002"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
<entryRelationship typeCode="SUBJ">
<!-- No Known Allergies -->
<!-- The negationInd = true negates the observation/value -->
<!-- The use of negationInd corresponds with the newer Observation.valueNegationInd -->
<observation classCode="O BS" moodCode="E VN" negationInd="true">
<!-- allergy - intolerance observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<templateId root="2.16.840.1.113883.10.20.22.4.7" extension="2014-06- 09"/>
<id root="4adc1020-7b14-11db-9fe1-0800200c9a66"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<text>
<reference value="#allergiesNoKnown"/>
</text>
<statusCode code="completed"/>
<!-- N–A - author/time records when this assertion was made -->
<effectiveTime>
<low nullFlavor="NA"/>
</effectiveTime>
<value xsi:type="CD" code="419199007"
displayName="Allergy to substance (disorder)"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"/>
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<time value="20100103"/>
<assignedAuthor>
<id extension="99999999" root="2.16.840.1.113883.4.6"/>
<code code="207Q00000X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="Health Care Provider Taxonomy"
displayName="Family Medicine"/>
<telecom use="WP" value="tel:555-555-1002"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
<!-- In C-CDA R2.1 the participant is required. -->
<participant typeCode="CSM">
<participantRole classCode="MANU">
<playingEntity classCode="MMAT">
<code nullFlavor="NA"/>
</playingEntity>
</participantRole>
</participant>
</observation>
</entryRelationship>
</act>
</entry>
</section>
This guidance for allergy observations is needed because mismatches between clinical statement patterns and source information pose challenges for implementers. Many EHR systems don't organize allergy data in this manner, creating implementation difficulties.
| Entry Template | Allergy - Intolerance Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.7:2014-06-09 (open)] |
|---|---|
| Template | Allergy - Intolerance Observation |
| Purpose | This template reflects a discrete observation about a patient's allergy or intolerance. |
| ActStatus | This is a discrete observation documented with a "completed" statusCode. The effectiveTime, or clinically relevant time, indicates when the observation holds true for the patient. For instance, if a provider notes a penicillin allergy history from five years ago during a current clinic visit, the effectiveTime is five years ago. It serves as the definitive indication of when the allergy-intolerance occurred. If resolved, an effectiveTime/high is present; if ongoing, it's not. |
| Negation | In this template, the negationInd attribute is used to indicate the absence of the allergy in observation/value (Observation.ValueNegationInd). |
| Other Considerations | The optional Allergy Status Observation template (identifier: urn:oid: 2.16.840.1.113883.10.20.22.4.28) signifies the status of the allergy-intolerance. Within the Allergy-Intolerance Observation, there is a Reaction Observation detailing the associated reaction, including its severity. Additionally, a Criticality Observation is included to indicate the seriousness of the allergy-intolerance, with possible values being high, low, or unable to assess criticality. Visit HL7 CDA Example Search for representing the expression “No Known Allergies. A negationInd of "true" coupled with an observation/value of SNOMED code 41919907. "Allergy to substance (disorder)" indicates that the patient has no known allergies. |
| Example | Example 42: Recording an allergy that started in January of 2009, but became a tracked concern as of January 4, 2014 |
| Example | Example 43: Updating an allergy that is no longer a concern |
| Example | Visit HL7 CDA Example Search for additional examples for allergies |
Table 47: Allergy - Intolerance Observation Template
Example 42: Recording an allergy that started in January of 2009, but became a tracked concern as of January 4, 2014
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<!-- ** Allergy problem act ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.30" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<id root="4a2ac5fc-0c85-4223-baee-c2e254803974" />
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<statusCode code="active"/>
<!-- This is the time stamp for when the allergy was first documented as a concern-->
<effectiveTime>
<low value="20140104123506-0500"/>
</effectiveTime>
<author>
<time value="20140104123506-0500"/>
...information identifying the author of the concern...
</author>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<!-- allergy observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<id root="4a2ac5fc-0c85-4223-baee-c2e254803974"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<!-- This is the time stamp for the clinical onset of the allergy. -->
<effectiveTime>
<low value="200901"/>
</effectiveTime>
...
</observation>
</entryRelationship>
</act>
</entry>
During an encounter, if a patient's record was updated to indicate that a penicillin allergy/intolerance concern noted one month ago, relating to an event five years ago, is no longer a concern, the CDA document would show:
Example 43: Updating an allergy that is no longer a concern
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<!-- ** Allergy problem act ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.30" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<id root="4a2ac5fc-0c85-4223-baee-c2e254803974" />
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<statusCode code="completed"/>
<!-- This is the time stamp for when the allergy was first documented as a concern-->
<effectiveTime>
<low value="20160104123506-0500"/>
<high value="20160204123506-0500"/>
</effectiveTime>
<author>
<time value="20160204123506-0500"/>
...information identifying the author of the concern...
</author>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<!-- allergy observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<id root="4a2ac5fc-0c85-4223-baee-c2e254803974"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<!-- This is the time stamp for the clinical onset of the allergy. -->
<effectiveTime>
<low value="200901"/>
</effectiveTime>
... Add representation of the Allergy Status Observation ...
</observation>
</entryRelationship>
</act>
</entry>
Medical Equipment includes devices implanted within the patient and devices the patient has or uses.
Each implanted device can be represented in an Individual Procedure Activity Procedure template.
Implanted devices can also go in a Medical Equipment Organizer template. Adding device info in procedure details doesn't replace listing it in the Medical Equipment section. Each part of the Medical Equipment Organizer has a Procedure Activity Procedure template.
UDI information is essential for interoperability. Content Creators should use a UDI Organizer in a Procedure Activity Procedure to represent parsed universal identifier data for implanted devices. Content Consumers should process this info when received.
| Entry Template | Procedure Activity Procedure [procedure: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.14:2014-06-09 (open)] |
|---|---|
| Template | Procedure Activity Procedure |
| Purpose | This template is used to represent any type of procedure from a surgical procedure to an education session. It may be used with a contained Product Instance template to represent a device implanted in or on a patient. In this case, targetSiteCode is used to record the location of the device in or on the patient's body. |
| ActStatus | This template has a state model with states: active, completed, aborted, and cancelled. When documenting an implanted medical device, use statusCode "completed." The effectiveTime, or clinically relevant time, represents when the procedure was performed, indicating when the device was implanted. Best practice is to use the TS data type to record a single point in time for the implantation. |
| Negation | In this template, the negationInd attribute is used to indicate the procedure was not performed. |
| Other Considerations | A Product Instance template is used template represents a specific device used in a patient or procedure. For device removal, use a separate Procedure Activity Procedure template with the device removal code. Use TS data type to record the removal time and link it to the original implant procedure using the Entry Reference template. To indicate a procedure was not performed, you may use the Indication template to explain the rationale. Multiple indication templates can be in a Procedure template. |
Example 44: Implanted Device – known procedure details
<component>
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.23"/>
<templateId root="2.16.840.1.113883.10.20.22.2.23" extension="2014- 06 - 09"/>
<code code="46264-8" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Medical Equipment"/>
<title>Implants</title>
<text>
<!-- table omitted for space -->
</text>
<entry>
<procedure classCode="PROC" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.14" extension="2014-06- 09"/>
<id extension="2744" root="1.2.840.114350.1.13.5552.1.7.2.737780"/>
<code code="609588000" codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"
displayName="Total knee replacement (procedure)">
<!-- Procedure is completed, even though the implant is still active -->
<statusCode code="completed"/>
<effectiveTime>
<!-- Placed Date -->
<low value="20160413"/>
</effectiveTime>
<targetSiteCode code="72696002" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT" displayName="Knee region structure">
<qualifier>
<name code="272741003" codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED
CT" displayName="laterality" />
<value code="7771000" codeSystem="2.16.840.1.113883.9.96" codeSystemName="SNOMED
CT" displayName="left" />
</qualifier>
</targetSiteCode>
<participant typeCode="DEV">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<id assigningAuthorityName="FDA"
extension="(01)00848486000400(11)160330(10)ABC634(21)123777" root="2.16.840.1.113883.3.3719"/>
<playingDevice>
<code nullFlavor="UNK">
<originalText>
<reference value="#implant1"/>
</originalText>
</code>
</playingDevice>
<!-- From Product Instance template:
The scopingEntity/id should correspond to FDA or the appropriate issuing
agency. -->
<scopingEntity>
<id root="2.16.840.1.113883.3.3719"/>
</scopingEntity>
</participantRole>
</participant>
</procedure>
</entry>
<entry>
<!-- example without qualifier omitted for space -->
</entry>
<entry>
<!-- example where targetSiteCode not mapped omitted for space -->
</entry>
</section>
</component>
Example 45: Additional Implanted Device
<component>
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.23"/>
<templateId root="2.16.840.1.113883.10.20.22.2.23" extension="2014-06- 09"/>
<code code="46264-8" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Medical Equipment"/>
<title>Implants</title>
<text>
<!-- table omitted for space -->
</text>
<entry>
<procedure classCode="PROC" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.14" extension="2014-06- 09"/>
<id extension="2744" root="1.2.840.114350.1.13.5552.1.7.2.737780"/>
<code nullFlavor="UNK"/>
<!-- Procedure is completed, even though the implant is still active -->
<statusCode code="completed"/>
<effectiveTime>
<!-- Placed Date -->
<low value="20160413"/>
</effectiveTime>
<participant typeCode="DEV">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<id assigningAuthorityName="FDA"
extension="(01)00848486000400(11)160330(10)ABC634(21)123777" root="2.16.840.1.113883.3.3719"/>
<playingDevice>
<code nullFlavor="UNK">
<originalText>
<reference value="#implant1"/>
</originalText>
</code>
</playingDevice>
<!-- From Product Instance template:
The scopingEntity/id should correspond to FDA or the appropriate issuing
agency. -->
<scopingEntity>
<id root="2.16.840.1.113883.3.3719"/>
</scopingEntity>
</participantRole>
</participant>
</procedure>
</entry>
<entry>
<!-- example without qualifier omitted for space -->
</entry>
<entry>
<!-- example where targetSiteCode not mapped omitted for space -->
</entry>
</section>
</component>
To declare that a patient has no implanted devices, the Medical Equipment section should be used that has a Procedure Activity Procedure entry with an effectiveTime that has a nullFlavor of ‘NA’ and a participantRole that has an id with a nullFlavor of ‘NA’ and a code of 40388003 – Implant. This combination states that the patient has not had a procedure to implant anything.
Example 46: No Implanted Devices
<component>
<section>
<!-- Medical equipment section -->
<templateId root="2.16.840.1.113883.10.20.22.2.23"/>
<templateId root="2.16.840.1.113883.10.20.22.2.23" extension="2014-06- 09"/>
<code code="46264-8" codeSystem="2.16.840.1.113883.6.1" />
<title>MEDICAL EQUIPMENT</title>
<!-- Alternative text: Patient has no history of procedures with implantable devices'-->
<!-- Alternative text: Patient has no implanted devices'-->
<text><paragraph ID="Proc">Patient has no history of implantable
devices</paragraph></text>
<entry>
<procedure classCode="PROC" moodCode="EVN" negationInd="true">
<!-- Procedure Activity Procedure -->
<templateId root="2.16.840.1.113883.10.20.22.4.14"/>
<templateId root="2.16.840.1.113883.10.20.22.4.14" extension="2014-06- 09"/>
<id root="d5b614bd-01ce-410d-8726-e1fd01dcc72a" />
<code code="71388002" codeSystem="2.16.840.1.113883.6.96"
displayName="Procedure"/>
<text>
<reference value="#Proc"/>
</text>
<statusCode code="completed" />
<effectiveTime nullFlavor="NA" />
<participant typeCode="DEV">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<!-- UDI is 'not applicable' since no procedure -->
<id nullFlavor="NA" root="2.16.840.1.113883.3.3719"/>
<playingDevice>
<code code="40388003" codeSystem="2.16.840.1.113883.6.96"
displayName="Implant"/>
</playingDevice>
<scopingEntity>
<id root="2.16.840.1.113883.3.3719"/>
</scopingEntity>
</participantRole>
</participant>
</procedure>
</entry>
</section>
</component>
Non-implanted devices are represented with the Non-Medicinal Supply Activity template. This template is used to represent devices the patient has such as eyeglasses or a cane.
| Entry Template | Non-Medicinal Supply [organizer: identifier urn: : hl7ii:2.16.840.1.113883.10.20.22.4.50:2014-06-09 (open)] )] |
|---|---|
| Template | Non-Medicinal Supply Activity |
| Purpose | This template represents equipment supplied to the patient (e.g., pump, inhaler, wheelchair, cane, eyeglasses, hearing aid). |
| ActStatus | This template uses a state model that includes the full range of status values defined in the ActStatus value set (2.16.840.1.113883.1.11.15933). The effectiveTime/low of the Non-Medicinal Supply act asserts when the person was first supplied with the indicated device. The effectiveTime/high asserts when the patient stopped using the supplied device. If the clinical statement is not about a specific non-medicinal device, it may be interpreted to generally describe when, for example, the patient first began wearing glasses or using a cane. If the clinical statement is about a specifically identified device, it may be interpreted to describe when the patient was supplied with that specific device. |
| Negation | Not specified. |
| Other Considerations | This template is not used when represented devices that are implanted within the patient. For information about how to represent implanted devices see the Procedure Activity Procedure template. |
| Example | Example 47: Non-Medicinal Supply – Cane and Eyeglasses |
Table 49: Non-Implanted Device Template
Example 47: Non-Medicinal Supply – Cane and Eyeglasses
<section>
<templateId root="2.16.840.1.113883.10.20.22.4.50" extension="2014-06- 09"/>
<code code="46264-8" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Medical Equipment"/>
<title>Medical Equipment</title>
<text>
<table>
<thead>
<tr>
<th>Medical Equipment</th>
<th>Device Identifier (if applicable)</th>
<th>Model / Serial / Lot (if applicable)</th>
<th>Date</th>
</tr>
</thead>
<tbody>
<tr ID="equipment1">
<td><content ID="equipment1item" styleCode="header">Eye Glasses</content></td>
<td>n/a</td>
<td>n/a</td>
<td>(01/13/2000 - )</td>
</tr>
<tr ID="equipment2">
<td><content ID="equipment2item" styleCode="header">Cane</content></td>
<td>n/a</td>
<td>n/a</td>
<td>(04/13/2016 - )</td>
</tr>
</tbody>
</table>
</text>
<entry>
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.50"/>
<templateId root="2.16.840.1.113883.10.20.22.4.50" extension="2014-06- 09"/>
<id extension="2744999" root="1.2.840.999.1.13.5552.1.7.2.99999"/>
<text><reference value="#equipment1"></reference></text>
<statusCode code="active"/>
<!-- low represents when person first received supply -->
<effectiveTime xsi:type="IVL_TS">
<low value="20000113"/>
<high nullFlavor="NI"/> <!-- patient use of supply is ongoing, could be omitted for the
same semantics. -->
</effectiveTime>
<!--<quantity value="1" /> -->
<participant typeCode="PRD">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<!-- id of supply is unknown; An FDA identifier is not required since this is a non-
implantable device -->
<id root="1.2.840.999.1.13.5552.1.7.2.999991" nullFlavor="UNK"/>
<playingDevice>
<code code="50121007" codeSystem="2.16.840.1.113883.6.96" displayName="Eyeglasses">
<originalText>
<reference value="#equipment1item"/>
</originalText>
</code>
</playingDevice>
<!-- Required by Product Instance Template -->
<scopingEntity>
<id nullFlavor="NA"/>
</scopingEntity>
</participantRole>
</participant>
</supply>
</entry>
<entry>
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.50"/>
<templateId root="2.16.840.1.113883.10.20.22.4.50" extension="2014-06- 09"/>
<id extension="2744999" root="1.2.840.999.1.13.5552.1.7.2.99999"/>
<!- - Template does not require a code, but best practice is to include a meaningful
code for the type of entry. -->
<code code="425399007" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMEDCT" displayName="Durable medical equipment (physical object)">
</code>
<text>
<reference value="#equipment2"></reference>
</text>
<statusCode code="active"/>
<!-- low represents when person first received supply -->
<effectiveTime xsi:type="IVL_TS">
<low value="20160413"/>
<high nullFlavor="NI"/> <!-- patient use of supply is ongoing -->
</effectiveTime>
<participant typeCode="PRD">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<!-- id of supply is unknown; An FDA identifier is not required since this is a
non-implantable device -->
<id root="1.2.840.999.1.13.5552.1.7.2.999991" nullFlavor="UNK"/>
<playingDevice>
<code code="87405001" codeSystem="2.16.840.1.113883.6.96" displayName="Cane">
<originalText>
<reference value="#equipment2item"/>
</originalText>
</code>
</playingDevice>
<!-- Required by Product Instance Template -->
<scopingEntity>
<id nullFlavor="NA"/>
</scopingEntity>
</participantRole>
</participant>
</supply>
</entry>
</section>
The Product Instance template is used to represent a particular device that was placed in a patient or used as part of a procedure or other act.
| Entry Template | Product Instance [participantRole: identifier urn:oid:2.16.840.1.113883.10.20.22.4.37] |
|---|---|
| Template | Product Instance |
| Purpose | This template is used to record the identifier and other details about the given product that was used. For example, it is important to have a record that indicates not just that a hip prostheses was placed in a patient but that it was a particular hip prostheses number with a unique identifier. |
| ActStatus | Not applicable. |
| Negation | Not applicable. |
| Other Considerations | The FDA Amendments Act specifies the creation of a Unique Device Identification (UDI) System that requires the label of devices to bear a unique identifier that will standardize device identification and identify the device through distribution and use. |
| Example | Example 44: Implanted Device – known procedure details |
| Example | Example 47: Non-Medicinal Supply – Cane and Eyeglasses |
Table 50: Product Instance Template
A Unique Device Identifier (UDI) is used to identify a device.
| Entry Template | UDI Organizer [organizer: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.312:2019-06-21 (open)] |
|---|---|
| Template | UDI Organizer |
| Purpose | This template is used to manage all the UDI-related templates to exchange the parsed UDI data elements and associated metadata including: device manufacturer, lot or batch number, serial number, manufacturing date, expiration date, distinct identification code, brand name, and model number, catalog number, company name, MRI safety, latex safety, and implantable device status. |
| ActStatus | The statusCode for the organizer is not specified. The effectiveTime element for the organizer is not specified. This template only conveys information about the UDI identifiers associated with a device. |
| Negation | Not specified. |
| Other Considerations | Only the device manufacturer information is required in this template. All other component observations are optional. If the implantable device status information is included, it SHALL contain one of following values from the NCI Thesaurus Code System (2.16.840.1.113883.3.26.1.1): - Active (C45329) - Inactive (C154407) - Malfunctioning (C122711) - Reduced Function (C160942) From the value set identified with OID 2.16.840.1.113762.1.4.1021.48 |
| ** Reference** | Product Instance, UDI Organizer, Device Identifier Observation |
| ** Reference** | See UDI Organizer for guidance on how to include an entryRelationship (typeCode=”COMP”) to reference a UDI Organizer containing the parsed components of the UDI identifier. The parsed components in the UDI Organizer shall align with the full UDI in the Product Instance. |
| Example | C-CDA Excamples Task Force Medical Equipment Section Examples |
Table 51: Unique Device Identifiers Template
The C-CDA Goal Observation entry template is designed to represent three different types of goals: patient goals, provider goals, and shared goals. The semantics to indicate if a goal is a patient, provider, or shared goal is represented in the author structure of the entry. The Progress Toward Goal observation is designed to sit inside the Goal and Outcome Observations to track the progress towards reaching a goal.
| Entry Template | Goal Observation [observation: identifier urn:oid:2.16.840.1.113883.10.20.22.4.121:2022-06-01 (open)] |
|---|---|
| Template | Progress Toward Goal Observation |
| Purpose | This template represents a patient health goal. A Goal Observation template may have related components that are acts, encounters, observations, procedures, substance administrations, or supplies. A goal may be a patient or provider goal. If the author is set to the recordTarget (patient), this is a patient goal. If the author is set to a provider, this is a provider goal. If both patient and provider are set as authors, this is a negotiated goal. A goal usually has a related health concern and/or risk. A goal may have components consisting of other goals (milestones). These milestones are related to the overall goal through entryRelationships. |
| ActStatus | Currently bound to the single concept of “active”. This has been reported as a limitation that needs to be addressed. |
| Negation | Not explicitly specified. |
| Other Considerations | This template current provides no guidance on what should be populated in the value element. A comment has been made against the design to request greater guidance be provided regarding the use of the value element in the design of the template. This template allows for multiple author participations ([0..*]). If the author is the recordTarget (patient), this is a patient goal. If the author is a provider, this is a provider goal. If both patient and provider are authors, this is a negotiated goal. If no author is present, it is assumed the document or section author(s) is the author of this goal. |
| Example | C-CDA R2.1 Figure 154 Goal Observation Example |
Table 52: Goal Observation Template Example task Force Goal examples Example 48: Goal Observation narrative
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.60"/>
<code code="61146-7" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Goals"/>
<title>ACTIVE GOALS</title>
<text>
<table>
<thead>
<tr>
<th>Patient Goal Type</th>
<th>Goal</th>
<th>Patient-Stated?</th>
<th>Author</th>
</tr>
</thead>
<tbody>
<tr>
<td>Blood Pressure</td>
<td>
<paragraph ID="goal1" styleCode="Bold">Get BP to normal (i.e. 120/80)</paragraph>
</td>
<td>No</td>
<td>Moreland, Andrew</td>
</tr>
...
</tbody>
</table>
</text>
<entry>
<observation classCode="OBS" moodCode="GOL">
<templateId root="2.16.840.1.113883.10.20.22.4.121"/>
<id extension="3241" root="1.2.840.114350.1.13.6289.1.7.2.737179"/>
<code nullFlavor="UNK">
<originalText>
<reference value="#goal2"/>
</originalText>
</code>
<statusCode code="active"/>
<effectiveTime>
<low value="20160412113717-0500"/>
</effectiveTime>
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<time value="20160412113717-0500"/>
<assignedAuthor>
<id extension="1" root="1.2.840.114350.1.13.6289.1.7.2.697780"/>
<assignedPerson>
<name>
<given>Andrew</given>
<family>Moreland</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
</observation>
</entry>
...
</section>
The Social History Observation template is a general template designed to represent a full range of social history observations. The Social History Observation Template has been updated to leverage Gravity Value Sets covering multiple social risk domains. See the Screening And Assessments page.
The Social History Observation template remains open to represent any SDOH observation related to conditions in which people live, learn, work, and play even if not defined in the Social Determinants of Health Conditions Value Set or in a specific template.
C-CDA Content Creators are encouraged to use specific templates over general templates when an appropriate specific template has been defined. |
| Entry Template | Social History Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.38:2022-06-01 (open)] |
|---|---|
| Template | Social History Observation |
| Purpose | This template represents a patient's occupations, lifestyle, and environmental health risk factors. Demographic data (e.g., marital status, race, ethnicity, religious affiliation) are captured in the header. |
| ActStatus | This template always has a statusCode of “completed.” This template does not include specific guidance about the meaning of the effectiveTime element. As an observation, the effectiveTime is the time at which the observation holds true for the patient. |
| Negation | Not explicitly specified. |
| Other Considerations | Additional more specific social history observation templates also exist. They constrain the Social History Observation in specific ways to represent key social history data elements that are essential for interoperable data exchange. Though tobacco use and exposure may be represented with a general Social History Observation template, it is recommended to use the Current Smoking Status template or the Tobacco Use template instead to aid in their exchange. |
| Example | Example 49: Social History Observation – Lead-Based paint in home environment illustrating use of the Social History Observation template for any social history observation |
Table 53: Entry Template
Example 49: Social History Observation - Lead-Based Paint in the Home
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.17"/>
<templateId root="2.16.840.1.113883.10.20.22.2.17" extension="2015-08- 01"/>
<code code="29762-2" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Social history"/>
<title>Social History</title>
<text>
<table>
<thead>
<tr>
<th>Information Type</th>
<th>Date</th>
<th>Relevant Information</th>
<th>Documented By</th>
</tr>
</thead>
<tbody>
<tr ID="SocialHistory_3">
<td>Characteristic of Residence</td>
<td><content>(04/12/2016)</content></td>
<td><content ID="SH3_SO"> Presence of lead-based paint (finding)
</content></td>
<td><content>M.Smith</content> <content>(04/12/2016)</content></td>
</tr>
</tbody>
</table>
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.38"/>
<templateId root="2.16.840.1.113883.10.20.22.4.38" extension="2015-08- 01"/>
<id extension="64020-Z9311" root="1.2.840.114350.1.13.6289.1.7.1.1040.1"/>
<code code="75274-1" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Characteristics of residence"/>
<text><reference value="#SocialHistory_3"></reference></text>
<statusCode code="completed"/>
<effectiveTime value="20160412"/>
<value xsi:type="CD" code="425203001 " displayName="Presence of lead-based paint (finding)"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMEDCT">
<originalText>
<reference value="#SH3_SO"></reference>
</originalText>
</value>
<author>
<time value="20160412"/>
<assignedAuthor>
<id extension="10.1" root="1.2.840.114350.1.1"/>
</assignedAuthor>
</author>
</observation>
</entry>
...
</section>
C-CDA Content Creators are strongly encouraged not to use the Smoking Status– Meaningful Use template to represent when the current smoking status started. |
| Entry Template | Smoking Status – Meaningful Use [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.78:2014-06-09 (open)] |
|---|---|
| Template | Smoking Status – Meaningful Use |
| Purpose | This template represents the current smoking status of the patient as specified in Meaningful Use (MU) Stage 2 requirements. |
| ActStatus | This template always has a statusCode of “completed.” This template represents a "snapshot in time" observation. It reflects the patient's smoking status at the time the observation is made. As a result, the effectiveTime is constrained to a time stamp that approximately corresponds with the author/time. |
| Negation | Not explicitly specified. |
| Other Considerations | This template requires use of the following range of possible answers: - Never smoked tobacco - Occasional tobacco smoker - Ex-smoker - Heavy tobacco smoker - Smokes tobacco daily - Smoker - Light tobacco smoker - Tobacco smoking consumption unknown Best practice implementation guidance allows alternate answers to be used. Within the Social History Section of a document there can be more than one Smoking Status observation recorded. The person’s “current” smoking status may have been recorded at several different points in time. |
| Example | Example 50: Unknown Smoking Status |
Table 54: Smoking Status
Example 50: Smoking Status
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.17"/>
<code code="29762-2" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Social History"/>
<title>Social History</title>
<text>
<list>
<caption>Smoking Status</caption>
<item>
<content>Unknown if ever smoked</content>
<content>As of: unknown</content>
<content>Recorded June 6, 2014 10:32am</content>
</item>
</list>
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.78"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low nullFlavor="UNK"/>
<value xsi:type="CD" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT" code="266927001" displayName="Tobacco smoking consumption unknown"/>
<author>
<time value="201406061032+0500"/>
<assignedAuthor>
<id root="2.16.840.1.113883.4.6" extension="99999999"/>
<code code="200000000X"
codeSystem="2.16.840.1.113883.6.101"
displayName="Allopathic and Osteopathic Physicians"/>
<telecom use="WP" value="tel:+1(555)555-1002"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
</observation>
</entry>
</section>
C-CDA Content Creators are strongly encouraged use the Tobacco Use template to describe dates associated with the patient's tobacco use over time. |
| Entry Template | Tobacco Use [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.85:2014-06-09 (open)] |
|---|---|
| Template | Tobacco Use |
| Purpose | This template contains information that describes a patient's tobacco use over time. |
| ActStatus | This template always has a statusCode of “completed.” The effectiveTime element is used to describe dates associated with the patient's tobacco use. It represents the clinically relevant time of the observation about the patient’s tobacco use. |
| Negation | Not explicitly specified. |
| Other Considerations | All the types of tobacco use from the tobacco use and exposure-finding hierarchy in SNOMED CT, including codes required for recording smoking status in Meaningful Use Stage 2 are used by this template. |
| Example | Example 51: Tobacco Use –Light Tobacco Smoker between 4/12/2010 and 4/12/2016. |
Table 55: Tobacco Use
Example 51: Tobacco Use –Light Tobacco Smoker between 4/12/2010 and 4/12/2016.
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.78"/>
<templateId root="2.16.840.1.113883.10.20.22.4.78" extension="2014-06- 09"/>
<id extension="64020-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.1040.1"/>
<code code="11367-0" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="History of Tobacco Use"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20100412"/>
<high value="20160412"/>
</effectiveTime>
<value xsi:type="CD" code="77176002" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMEDCT" displayName=" Light tobacco smoker (finding)"/>
</observation>
This observation represents the biological sex assigned to the patient at birth. This observation often reflects the sex that is entered on the person's birth certificate at time of birth.
| Entry Template | Birth Sex Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.200:2016-06-01 (open)] |
|---|---|
| Template | Birth Sex Observation |
| Purpose | This observation represents the sex of the patient at birth. It is often the sex that is entered on the person's birth certificate at time of birth. |
| ActStatus | This template always has a statusCode of “completed.” |
| Negation | Not explicitly specified. |
| Other Considerations | This observation is intended to be used in the Social History section. This observation is not appropriate for recording patient gender (administrativeGender). The patient’s administrative gender is recorded in the header of the document using the recordTarget.administrativeGender element. |
| Example | Example 52: Birth Sex |
Table 56: Birth Sex Template
Example 52: Birth Sex
<!-- Birth Sex Entry -->
<observation classCode="OBS" moodCode="EVN">
<!-- New templateId for Birth Sex -->
<!-- Not planning to assert conformance to Social History Observation due to different
vocab -->
<templateId root="2.16.840.1.113883.10.20.22.4.200" extension="2016-06- 01"/>
<code code="76689-9" codeSystem="2.16.840.1.113883.6.1"
displayName="Sex Assigned At Birth"/>
<text>
<reference value="#BSex_Narrative1"/>
</text>
<statusCode code="completed"/>
<!-- effectiveTime if present should match birthTime -->
<!-- Request name change to QRDA value set (2.16.840.1.113762.1.4.1) - ONC Birth Sex -->
<value xsi:type="CD" codeSystem="2.16.840.1.113883.5.1"
codeSystemName="AdministrativeGender"
code="F" displayName="Female">
<originalText>
<reference value="#BSex_value"/>
</originalText>
</value>
</observation>
The Pregnancy Status Observation is used to represent a person’s pregnancy status over time. It is a type of social history observation and can included in the Social History Section. For communicating more detailed observations related to an individual’s pregnancy status, implementers can also consider utilizing the templates in the HL7 CDA® R2 Implementation Guide: C-CDA R2.1 Supplemental Templates for Pregnancy Status, Release 1 - US Realm.
| Entry Template | Pregnancy Status Observation [observation: identifier urn:oid:2.16.840.1.113883.10.20.15.3.8 (open)] |
|---|---|
| Template | Pregnancy Status Observation |
| Purpose | This template contains information that describes a patient's pregnancy status over time. |
| ActStatus | This template always has a statusCode of “completed.” The effectiveTime element is used to describe dates associated with the patient's different pregnancy statuses over time. It represents the clinically relevant time of the observation about the patient’s pregnancy status. |
| Negation | Not explicitly specified. |
| Other Considerations | The value element of this template holds the patient’s pregnancy status. Possible coded concepts for this data element include: - Possible pregnancy (finding) - Not pregnant (finding) - Pregnant (finding) |
| Example | Example 53: Patient was pregnant from 4/10/2011 to 1/12/2012 |
| Example | Example 54: Patient was not pregnant |
| Example | Example 55: Unknown if the patient was pregnant or not |
Table 57: Pregnancy Status Observation
Example 53: Patient was pregnant from 4/10/2011 to 1/12/2012
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.15.3.8"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
<high value="20120112"/>
</effectiveTime>
<value xsi:type="CD" code="77386006"
displayName="pregnant"
codeSystem="2.16.840.1.113883.6.96"/>
<entryRelationship typeCode="REFR">
<templateId root="2.16.840.1.113883.10.20.15.3.1"/>
... </entryRelationship>
</observation>
To indicate that the patient was not pregnant during a specified date range, the Pregnancy Status Observation entry should also be used, but with a negationInd set to “true” to indicate that the patient was not pregnant during the date range specified by the effectiveTime element.
Example 54: Patient was not pregnant
<observation classCode="OBS" moodCode="EVN" negationInd="true">
<templateId root="2.16.840.1.113883.10.20.15.3.8"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
<high value="20110710"/>
</effectiveTime>
<value xsi:type="CD" code="77386006"
displayName="pregnant"
codeSystem="2.16.840.1.113883.6.96"/>
<entryRelationship typeCode="REFR">
<templateId root="2.16.840.1.113883.10.20.15.3.1"/>
... </entryRelationship>
</observation>
Finally, to indicate that it was unknown if the patient was pregnant or not, then a nullFlavor should be used on the observation to indicate that the patient’s pregnancy status was unknown. An effectiveTime element can be included to assert the period over which it was unknown.
Example 55: Unknown if the patient was pregnant or not
<observation classCode="OBS" moodCode="EVN" nullFlavor="UNK">
<templateId root="2.16.840.1.113883.10.20.15.3.8"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
<high value="20110710"/>
</effectiveTime>
<value xsi:type="CD" code="77386006"
displayName="pregnant"
codeSystem="2.16.840.1.113883.6.96"/>
<entryRelationship typeCode="REFR">
<templateId root="2.16.840.1.113883.10.20.15.3.1"/>
... </entryRelationship>
</observation>
Results generated by laboratories, imaging procedures, and other procedures are coded as result observations and contained within a Results Organizer. The Result Organizer.code element is used to categorize the contained results into one of several commonly accepted values (e.g., “Hematology”, “Chemistry”, “Nuclear Medicine”). This is how laboratory tests are distinguished from Diagnostic imaging tests.
Pathology is the superset domain that encompasses several subdisciplines: anatomic pathology, chemical pathology, clinical pathology, forensic pathology, genetic pathology, hematology, immunopathology, etc.(Pathology Tests Definition). Therefore, a laboratory test is a type of pathology test.
This template provides a mechanism for grouping result observations. It contains information applicable to all of the contained result observations. If any Result Observation within the organizer has a statusCode of "active", the Result Organizer must also have a statusCode of "active."
The Result Organizer.code element is used to categorize the contained results. This element is encouraged to be populated with a LOINC code that defines a specific test panel (e.g., “CBC W Auto Differential panel - Blood“) or general type of testing (e.g., “Hematology”, “Chemistry”, “Nuclear Medicine”). |
| Entry Template | Result Organizer [organizer: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.1:2015-08-01 (open)] |
|---|---|
| Template | Result Organizer |
| Purpose | This template provides a mechanism for grouping result observations. It contains information applicable to all of the contained result observations. |
| ActStatus | If any Result Observation within the organizer has a statusCode of "active", the Result Organizer must also have a statusCode of "active". The range of time specified in the Result Organizer/effectiveTime element should encompass the lowest effectiveTime/low and the highest effectiveTime/high for the Result Observations within the organizer. |
| Negation | Not specified. |
| Other Considerations | If the Author Participation template is specified for the Organizer, this context applies to all the component observations unless a component observation includes a different Author Participation template.) |
| Example | Example 56: Result Organizer for CBC W Auto Differential panel - Blood |
Table 58: Result Organizer Template
Example 56: Result Organizer for CBC W Auto Differential panel - Blood
C-CDA Example Task Force Result Section examples
<section>
<!-- Results Section -->
<!-- This example shows that laboratory results more recent than the inpatient encounter are
available at Good Health Hospital at the time the CCD is generated. This is a Patient Summary,
not an Encounter Document. It covers a span of time.-->
<templateId root="2.16.840.1.113883.10.20.22.2.3.1" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.2.3.1"/>
<code code="30954-2" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Relevant diagnostic tests/laboratory data Narrative"/>
<title>RESULTS</title>
<text>
<content ID="Results_STR">This document includes patent laboratory results for testing
performed within the past three months.</content> <br></br>
<br></br>
<content styleCode="Bold">CBC W Auto Differential panel - Blood (04/01/2016)</content> |
<content>Diagnostic Labs (04/02/2016)</content>
<table>
<thead>
<tr>
<th>Result Type</th>
<th>Result Value</th>
<th>Relevant Reference Range</th>
<th>Interpretation</th>
<th>Date</th>
</tr>
</thead>
<tbody>
<tr>
<td ID="result1">Hemoglobin</td>
<td ID="resultvalue1">13.2 g/dL</td>
<td ID="referencerange1">Normal range for women is 12.0 to 15.5 grams per
deciliter</td>
<td>Normal</td>
<td>04/01/2016</td>
</tr>
<tr>
<td ID="result2">Leukocytes</td>
<td ID="resultvalue2">6.7 10*3/uL</td>
<td ID="referencerange2">Normal white blood cell count range 3.5-10.5 billion
cells/L</td>
<td>Normal</td>
<td>04/01/2016</td>
</tr>
...
</tbody>
</table>
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<!-- Section Time Range Observation -->
<templateId root="2.16.840.1.113883.10.20.22.4.201" extension="2016-06- 01"/>
<code code="82607-3" displayName="Clinical data [Date and Time Range]"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"></code>
<text><reference value="#Results_STR"></reference></text>
<statusCode code="completed"/>
<value xsi:type="IVL_TS">
<low value="20160401"/>
<high value="20160412"/>
</value>
<author>
<!-- The system that generated the CCD -->
<time value="20160412114559-0500"/>
<assignedAuthor>
<id extension="8.3" root="1.2.840.114350.1.1"/>
</assignedAuthor>
</author>
</observation>
</entry>
<entry typeCode="DRIV">
<organizer classCode="BATTERY" moodCode="EVN">
<!-- ** Result organizer ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.1" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.1"/>
<id root="7d5a02b0-67a4-11db-bd13-0800200c9a66"/>
<code code="57021-8"
displayName="CBC W Auto Differential panel - Blood"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20160401102500-0500"/>
<high value="20160402121500-0500"/>
</effectiveTime>
<!-- author at the organizer-level conducts down into the component observations.-->
<author>
<time value="20160402121500-0500"/>
<assignedAuthor>
<id extension="333444444" root="2.16.840.1.113883.4.6"/>
<code code="246Q00000X" displayName="Technician, Pathology"
codeSystem="2.16.840.1.113883.6.101" codeSystemName="Healthcare Provider Taxonomy
(HIPAA)"/>
<representedOrganization>
<name>Diagnostic Labs</name>
</representedOrganization>
</assignedAuthor>
</author>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- ** Result observation ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.2" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.2"/>
<id root="107c2dc0-67a5-11db-bd13-0800200c9a66"/>
<code code="718-7" displayName="Hemoglobin" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<text>
<reference value="#result1"/>
</text>
<statusCode code="completed"/>
<effectiveTime value="20160401102500-0500"/>
<value xsi:type="PQ" value="13.2" unit="g/dL"/>
<interpretationCode code="N" codeSystem="2.16.840.1.113883.5.83"/>
<referenceRange>
<observationRange>
<value xsi:type="IVL_PQ">
<low value="12.0" unit="g/dL"/>
<high value="15.5" unit="g/dL"/>
</value>
</observationRange>
</referenceRange>
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- ** Result observation ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.2" extension="2015-08- 01"/>
<templateId root="2.16.840.1.113883.10.20.22.4.2"/>
<id root="a69b3d60-2ffd-4440-958b-72b3335ff35f"/>
<!-- This is the correct long common name display name per LOINC web and per to 2000
-->
<code code="6690-2" displayName="Leukocytes[#/volume] in Blood by Automated count"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<text>
<reference value="#result2"/>
</text>
<statusCode code="completed"/>
<effectiveTime value="20160401102500-0500"/>
<value xsi:type="PQ" value="6.7" unit="10*3/uL"/>
<interpretationCode code="N" codeSystem="2.16.840.1.113883.5.83"/>
<referenceRange>
<observationRange>
<value xsi:type="IVL_PQ">
<low value="4.3" unit="10*9/L"/>
<high value="10.8" unit="10*9/L"/>
</value>
</observationRange>
</referenceRange>
</observation>
</component>
...
</organizer>
</entry>
</section>
| Entry Template | Result Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.2:2023-05-01 (open)] |
|---|---|
| Template | Result Observation |
| Purpose | This template represents the results of a laboratory, radiology, or other study performed on a patient. |
| ActStatus | The result observation includes a statusCode to allow recording the status of an observation. “Pending” results (e.g., a test has been run but results have not been reported yet) should be represented as “active” ActStatus. The effectiveTime element represents the clinically relevant time of the measurement (e.g., the time a blood pressure reading is obtained, the time the blood sample was obtained for a chemistry test). |
| Negation | Not specified. To indicate that a test was not performed use the Procedure Activity Procedure template with the negationInd attribute of “true.” |
| Other Considerations | If the Author Participation template is specified for the Organizer, this context applies to all the component observations unless a component observation includes a different Author Participation template. If any Result Observation within the organizer has a statusCode of "active," the Result Organizer must also have a statusCode of "active." The range of time specified in the Result Organizer/effectiveTime element should encompass the lowest effectiveTime/low and the highest effectiveTime/high for the Result Observations within the organizer. |
| Example | Example 56: Result Organizer for CBC W Auto Differential panel - Blood |
Table 59: Result Observation Template
| Entry Template | Vital Signs Organizer [organizer: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.26:2015-08-01 (open)] |
|---|---|
| Template | Vital Signs Organizer |
| Purpose | This template provides a mechanism for grouping vital signs (e.g., grouping systolic blood pressure and diastolic blood pressure, BMI, body height, body weight). |
| ActStatus | A Vital Signs Organizer SHALL have a statusCode of "completed". The effectiveTime may be a timestamp or an interval that spans the effectiveTimes of the contained vital signs observations. |
| Negation | Not specified. |
| Other Considerations | If the Author Participation template is specified for the Organizer, this context applies to all the component observations unless a component observation includes a different Author Participation template. |
| Example | Example 57: Vital Signs Organizer |
Table 60: Vital Signs Organizer Template C-CDA Examples Task Force Vital Sign Section examples Example 57: Vital Signs Organizer
<section>
<!-- This section shows an example of rendering information in the correct local time, which
recording it in the discrete data using a UTC offset. -->
<!-- Note that in June, UTC offset -0500 is Central time. and Eastern Timezone is the local
time for this example. -->
<templateId root="2.16.840.1.113883.10.20.22.2.4.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.4.1" extension="2015- 08 - 01"/>
<templateId root="2.16.840.1.113883.10.20.22.2.4.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.4.1" extension="2015-08- 01"/>
<code code="8716-3" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Vital Signs"/>
<title>LAST RECORDED VITAL SIGNS</title>
<text>
<table>
<thead>
<tr>
<th>Vital Sign</th>
<th>Reading</th>
<th>Time Taken</th>
</tr>
</thead>
<tbody>
<tr>
<td styleCode="cellHeader">Blood Pressure</td>
<td>
<content ID="sysBP_5523355980">140</content>/<content
ID="diaBP_5523355980">90</content>
</td>
<td>(06/25/2015 1:33pm EST)</td>
</tr>
<tr>
<td styleCode="cellHeader">Weight</td>
<td ID="weight_5523355980">83.9 kg (185 lb)</td>
<td>(06/25/2015 1:33pm EST)</td>
</tr>
</tbody>
</table>
</text>
<entry typeCode="DRIV">
<organizer classCode="CLUSTER" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.26"/>
<templateId root="2.16.840.1.113883.10.20.22.4.26" extension="2015-08- 01"/>
<id extension="5523355980-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.2109"/>
<code code="46680005" codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED CT"
displayName="Vital signs">
<translation code="74728-7" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Vital signs, weight, height, head circumference, oximetry, BMI, and BSA
panel"/>
</code>
<statusCode code="completed"/>
<effectiveTime value="20160625123300-0500"/>
<!-- Context Conduction permits the author to be included once at the organizer level.
Authorship conducts down into the component observations. -->
<author>
<time value="20160625123300-0500"/>
<assignedAuthor>
<id extension="811111111" root="2.16.840.1.113883.4.6"/>
</assignedAuthor>
</author>
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.27"/>
<templateId root="2.16.840.1.113883.10.20.22.4.27" extension="2014-06- 09"/>
<id extension="5523355980-sysBP-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.2109.1"/>
<code code="8480-6" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="SYSTOLIC BLOOD PRESSURE"/>
<text>
<reference value="#sysBP_5523355980"/>
</text>
<statusCode code="completed"/>
<effectiveTime value="20160625123300-0500"/>
<value xsi:type="PQ" unit="mm[Hg]" value="140"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"/>
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.27"/>
<templateId root="2.16.840.1.113883.10.20.22.4.27" extension="2014-06- 09"/>
<id extension="5523355980-diaBP-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.2109.1"/>
<code code="8462-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="DIASTOLIC BLOOD PRESSURE"/>
<text>
<reference value="#diaBP_5523355980"/>
</text>
<statusCode code="completed"/>
<effectiveTime value="20160625123300-0500"/>
<value xsi:type="PQ" unit="mm[Hg]" value="90"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"/>
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.27"/>
<templateId root="2.16.840.1.113883.10.20.22.4.27" extension="2014-06- 09"/>
<id extension="5523355980-weightC83-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.2109.1"/>
<code code="29463-7" codeSystem="2.16.840.1.113883.6.1" displayName="Body weight"/>
<text>
<reference value="#weight_5523355980"/>
</text>
<statusCode code="completed"/>
<effectiveTime value="20160625123300-0500"/>
<value xsi:type="PQ" unit="kg" value="83.915"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"/>
</observation>
</component>
</organizer>
</entry>
</section>
| Entry Template | Vital Sign Observation [observation: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.27:2014-06-09 (open)] |
|---|---|
| Template | Vital Sign Observation |
| Purpose | This template represents measurements of common vital signs. |
| ActStatus | The result observation includes a statusCode to allow recording the status of an observation. “Pending” results (e.g., a test has been run but results have not been reported yet) should be represented as “active” ActStatus. The effectiveTime element represents the clinically relevant time of the measurement (e.g., the time a blood pressure reading is obtained, the time the blood sample was obtained for a chemistry test). |
| Negation | Not specified. To indicate that a test was not performed use the Procedure Activity Procedure template with the negationInd attribute of “true.” Reference: 5.2.15 Procedure |
| Other Considerations | If the Author Participation template is specified for the Organizer, this context applies to all the component observations unless a component observation includes a different Author Participation template. Vital Signs Observations require standard units to be used when recording a particular value. The Vital Sign Result Type value set includes method-less types of vital sign observations. When method-specific vital sign measures are used, a more specific LOINC code can be used in the translation element of the vital sign observation code element. |
| Example | Example 57: Vital Signs Organizer |
| Example | Example 58: Vital Sign Coding with Translation |
Table 61: Vital Signs Observation Template
| Vital Sign | LOINC Code | Unit of Measure |
|---|---|---|
| Body height | 8302-2 | cm |
| Head Occipital-frontal circumference | 9843-4 | cm |
| Body Weight | 29463-7 | kg |
| Body Temperature | 8310-5 | Cel |
| Systolic blood pressure | 8480-6 | Mm[Hg] |
| Diastolic blood pressure | 8462-4 | Mm[Hg] |
| Respiratory Rate | 9279-1 | /min |
| Body mass index (BMI) [Ratio] | 39156-5 | kg/m2 |
| Body surface area Derived from formula | 3140-1 | m2 |
| Heart Rate (synonym for Pulse) | 8867-4 | /min |
| Inhaled Oxygen concentration | 3151-8 | liters/min |
| Oxygen Saturation in Arterial blood | 2708-6 | % |
| Additional concept codes often needed as a translation to a method-specific measure | ||
| Oxygen saturation in Arterial blood by Pulse oximetry | 59408-5 | % |
| Heart Rate by Pulse oximetry | 8889-8 | /min |
** Table 62: Vital Sign Observation LOINC Codes and Units for Essential Vital Sign Data Elements**
Example 58: Vital Sign Coding with Translation
<code code="29463-7" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Body weight">
<translation code="8350-1" codeSystem="2.16.840.1.113883.6.1"
displayName="Body weight Measured - with clothes" />
</code>
The Medication Activity Entry template is used to record a medication that has been administered and also is used to record statements about medications being taken. These two clinical statement patterns are identical, so the semantics is discerned through the context of use. Both are represented as a Medications with a substanceAdministration/@moodCode="EVN". A statement of this type can be interpreted to represent an actual administration of the medication. It also can be used to make a statement about the medication a patient takes.
The Admission Medication and Discharge Medication entry templates include a structural context around the Medication Activity Entry template. The additional structure includes semantic coding that identifies the medication information as admission or discharge medication information.
Example 59: Medication Administration
<substanceAdministration classCode="SBADM" moodCode="EVN">
<!-- This medication use case is a medication administered a single time in the past.-->
<templateId root="2.16.840.1.113883.10.20.22.4.16" />
<id root="1061a257-3b5c-4b09-9dc7-23e59b788b18"/>
<text>
<reference value="#Medication_7" />
</text>
<statusCode code="completed"/>
<effectiveTime xsi:type="TS" value="201309111603-0700"/>
<routeCode code="C38288" codeSystem="2.16.840.1.113883.3.26.1.1" displayName="ORAL"
codeSystemName="National Cancer Institute Thesaurus" />
<doseQuantity value="2" />
<consumable>
<manufacturedProduct classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.23" />
<manufacturedMaterial>
<code code="243670" codeSystem="2.16.840.1.113883.6.88"
displayName="Aspirin 81 MG Oral Tablet">
<originalText>
<reference value="#MedicationName_7"/>
</originalText>
</code>
</manufacturedMaterial>
</manufacturedProduct>
</consumable>
</substanceAdministration>
Medication activities with substanceAdministration/@moodCode= "INT" document what a clinician intends a patient to be taking. For example, a clinician may intend that a patient begin taking Lisinopril 20 mg PO for blood pressure control. The Planned Medication Activity entry can also be used to record a medication that the physician intends the patient to take at some time in the future.
Example 60: Planned Medication
<substanceAdministration classCode="SBADM" moodCode="INT">
<!-- This medication use case is a medication that is to be administered.-->
<templateId root="2.16.840.1.113883.10.20.22.4.16" />
<id root="1061a257-3b5c-4b09-9dc7-23e59b788b18"/>
<text>
<reference value="#Medication_7" />
</text>
<statusCode code="active"/>
<!-- This first effectiveTime shows that medication was prescribed on January 18, 2014
(not known to have stopped)-->
<effectiveTime>
<low value="20140118"/>
<high nullFlavor="NI"/>
</effectiveTime>
<!-- This second effectiveTime represents every 4-6 hours from medication sig. -->
<!-- Operator "A" means that this timing information is in addition to previous
effectiveTime information provided-->
<effectiveTime xsi:type="PIVL_TS" operator="A">
<period xsi:type="IVL_PQ">
<low value="4" unit="h"/>
<high value="6" unit="h"/>
</period>
</effectiveTime>
<routeCode code="C38288" codeSystem="2.16.840.1.113883.3.26.1.1" codeSystemName="NCI
Thesaurus" displayName="Oral"/>
<!-- This relates directly to the code used for medication. Since it's a tablet, then
only specified needed in dose is 2x per administration-->
<doseQuantity value="2"/>
<consumable>
<manufacturedProduct classCode="MANU">
<!-- ** Medication information ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.23"/>
<id root="0be61984-eaa5-46b3-b75b-1d1ba28b5fff"/>
<manufacturedMaterial>
<!-- Medications should be specified at a level corresponding to prescription when
possible, such as 30mg oral tablet (branded)-->
<code code="1049529" displayName="Sudafed 30 MG Oral Tablet"
codeSystem="2.16.840.1.113883.6.88" codeSystemName="RxNorm">
<originalText>
<reference value="#MedicationName_1"/>
</originalText>
</code>
</manufacturedMaterial>
</manufacturedProduct>
</consumable>
</substanceAdministration>
The Medication Supply Order entry records activities associated with ordering medications. The Medication Dispense entry records when medications are dispensed to the patient.
Example 61: Medication Dispense
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.18" extension="2014-06- 09" />
<id root="1.2.3.4.56789.1" extension="cb734647-fc99-424c-a864-7e3cda82e704" />
<statusCode code="completed" />
<effectiveTime value="201208151450-0800" />
<repeatNumber value="1" />
<quantity value="75" />
<product>
<manufacturedProduct classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.23" extension="2014-06- 09" /> ...
</manufacturedProduct>
</product>
<performer>
<assignedEntity>
...
</assignedEntity>
</performer>
</supply>
The structure of a medication entry can be complex. It is complicated by the fact that any one of these templates may include other types of medication templates within additional entryRelationships. To support interoperability, implementers should minimize the amount of template nesting used to express medication information.
When representing medications, consideration needs to be given to the way date/time intervals are represented. See Chapter 5.1.10.2 Date/Time Precision for additional information about how to represent and interpret date ranges that use an effectiveTime/low and effectiveTime/high. The CDA Example Task Force includes a document that summarizes commonly used medication frequencies.
C-CDA Examples Task Force Medication Examples
When recording an actual immunization (with moodCode = EVN), the effectiveTime represents when the immunization was given, and this will generally just be a single dateTime value. Most of the time, when recording the immunization date, the effectiveTime element should contain just a single @value. However, there is a use case for using an interval when requesting an immunization, i.e. have this immunization done between date 1 and date 2.
C-CDA Examples Task Force Immunization Examples
<section>
<!-- Immunizations section -->
<templateId root="2.16.840.1.113883.10.20.22.2.2.1"/>
<code code="11369-6" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="History of immunizations"/>
<title>IMMUNIZATIONS</title>
<text>
<content ID="immunSect"/>
<!-- table omitted for space -->
</text>
<entry typeCode="DRIV">
<substanceAdministration classCode="SBADM" moodCode="EVN" negationInd="false">
<!-- ** Immunization activity ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.52"/>
<id root="e6f1ba43-c0ed-4b9b-9f12-f435d8ad8f92"/>
<text>
<reference value="#immun1"/>
</text>
<!-- Indicates the status of the substanceAdministartion -->
<statusCode code="completed"/>
<effectiveTime value="20100815"/>
<consumable>
<manufacturedProduct classCode="MANU">
<!-- ** Immunization medication information ** -->
<templateId root="2.16.840.1.113883.10.20.22.4.54"/>
<manufacturedMaterial>
<code code="88" codeSystem="2.16.840.1.113883.12.292"
displayName="Influenza virus vaccine" codeSystemName="CVX"/>
<lotNumberText>1</lotNumberText>
</manufacturedMaterial>
<!-- Optional manufacturerOrganization -->
<manufacturerOrganization>
<name>Health LS - Immuno Inc.</name>
</manufacturerOrganization>--> </manufacturedProduct>
</consumable>
<!-- Optional Performer -->
<performer>
<assignedEntity>
<id root="2.16.840.1.113883.19.5.9999.456" extension="2981824"/>
<addr>
<streetAddressLine>1021 Health Drive</streetAddressLine>
<city>Ann Arbor</city>
<state>MI</state>
<postalCode>99099</postalCode>
<country>US</country>
</addr>
<telecom nullFlavor="UNK"/>
<assignedPerson>
<name>
<given>Amanda</given>
<family>Assigned</family>
</name>
</assignedPerson>
<representedOrganization>
<id root="2.16.840.1.113883.19.5.9999.1394"/>
<name>Good Health Clinic</name>
<telecom nullFlavor="UNK"/>
<addr nullFlavor="UNK"/>
</representedOrganization>
</assignedEntity>
</performer> --> </substanceAdministration>
</entry>
</section>
When recording the immunization status code, the normal value would be “completed”, as this represents an immunization that has been completely given. In extremely rare circumstances, a status of “active” could be used. The use of ”active” implies that a single immunization is still on-going. This would not be appropriate for one shot in a series of immunizations. Series immunizations should be represented with multiple Immunization Activity entries.
Historically, C-CDA, up to version 2.1, aligned with the HL7 v3 RIM definition of a procedure as an act that alters the physical condition of the subject. It defined three procedure types using the Observation and Act classes for procedures that do not alter the subject's physical condition. Industry uses the procedure template design regardless of procedure type, which is contained in C-CDA as the Procedure Activity Procedure Template. This template records current and historical procedures performed on or for a patient, encompassing various activities like surgical, diagnostic, endoscopic procedures, counseling, physiotherapy, and more. These procedures can be performed by healthcare professionals, service providers, friends or relatives, or even the patients themselves.
When representing that a procedure was not performed, the Indication (identifier: urn:hl7ii:2.16.840.1.113883.10.20.22.4.19:2023-05-01 template is encouraged to be used to represent the rationale for not performing the procedure. More than one indication template may be contained within a Procedure template. |
| Entry Template | Procedure Activity Procedure [procedure: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.14:2022-06-01 (open)] |
|---|---|
| Template | Procedure Activity Procedure |
| Purpose | This template records current and historical procedures performed on or for a patient, encompassing various activities like surgical, diagnostic, endoscopic procedures, counseling, physiotherapy, and more. These procedures can be performed by healthcare professionals, service providers, friends or relatives, or even the patients themselves. |
| ActStatus | The template includes a state model that includes active, completed, aborted, and cancelled. The effectiveTime, also referred to as the “clinically relevant time” is the time at which the procedure was performed. If the status of a procedure was active at the time a C-CDA document was created, the effectiveTime/low would indicate the date/time the procedure was started and the effectiveTime/high SHOULD not be present. If the status of a procedure was completed, aborted or cancelled, the effectiveTime/high SHOULD be populated. Implementer best practice would be use of the TS_IVL data type. For implementers who are not able to represent a time interval, effectiveTime/value MAY be populated. |
| Negation | In this template, the negationInd attribute is used to indicate the procedure was not performed. |
| Other Considerations | When representing that a procedure was not performed, the Indication (identifier: urn:hl7ii:2.16.840.1.113883.10.20.22.4.19:2014-06-09 template MAY be used to represent the rationale for not performing the procedure. More than one indication template may be contained within a Procedure template. |
| Example | How to represent Procedure Activity Procedure |
An assessment is a collection of observations that together yield a summary evaluation of a particular condition. Examples include the Braden Scale (assesses pressure ulcer risk), APACHE Score (estimates mortality in critically ill patients), Mini-Mental Status Exam (assesses cognitive function), APGAR Score (assesses the health of a newborn), and Glasgow Coma Scale (assesses coma and impaired consciousness). This template is designed to represent both the question or type of information assessed as well as the associated answer/result.
The Assessment Scale Observation Template is encouraged to be used to represent the content of the Assessment. |
| Entry Template | Assessment Scale Observation [observation: identifier urn:oid:2.16.840.1.113883.10.20.22.4.69:2022-06-01 (open)] |
|---|---|
| Template | Assessment Scale Observation |
| Purpose | An assessment scale is a collection of observations that together yield a summary evaluation of a particular condition. |
| ActStatus | This template always has a statusCode of “completed”. The effectiveTime represents the clinically relevant time of the measurement, which may be when the assessment was performed. |
| Negation | Not specified. |
| Other Considerations | The observation/value element represents the “answer” or result for the assessed “question” or measurement. The clinical statement may also include an interpretation and may include multiple reference ranges. Additionally, each assessment scale observation may contain zero or more Assessment Scale Supporting Observations. This structure allows the Assessment Scale Observation to mirror the format of many assessment tools. An Assessment Scale Supporting Observation represents the components of a scale used in an Assessment Scale Observation. The individual parts that make up the component may be a group of cognitive or functional status observations. |
| Example | PHQ-9 |
| Example | WE CARE |
| Example | Glasgow Coma |
Table 64: Assessment Scale Observation Template
Note Activity entries provide structured information for notes found in a Notes Section or designated sections in clinical note documents, making the human-readable narrative notes more machine-processable.
C-CDA Content Creators are encouraged to not use the Note Activity template in place of a more specific C-CDA entry template. |
| Entry Template | Note Activity [act: identifier urn:hl7ii:2.16.840.1.113883.10.20.22.4.202:2016-11-01 (open)]] |
|---|---|
| Template | Note Activity |
| Purpose | The Note Activity represents a clinical note. |
| ActStatus | This template always has a statusCode of “completed.” The effectiveTime represents the clinically relevant time of note, which may be when the note was written. |
| Negation | Not specified. |
| Other Considerations | Similar to Comment Activity, Note Activity allows for specific coding of note content, ensuring relevance to the document's message. It can augment data in a related standard C-CDA entry using entryRelationship, such as adding it to a Procedure Activity Procedure or an Encounter Activity in the Encounters Section. The Note Activity template can also stand alone in a C-CDA section (e.g., a note about procedures in the Procedures Section) or enhance a narrative-only section like Hospital Course. When the data type is unknown or no single section fits, place the Note Activity in a general Notes Section, such as a free-text consultation note or one with subjective, objective, assessment, and plan information combined. The examples below illustrate various clinical note representations using the Note Activity template. |
Table 65: Note Activity Template
Example 63: Note Activity as entryRelationship to C-CDA Entry.
<section>
<!-- C-CDA Procedures Section -->
<templateId root="2.16.840.1.113883.10.20.22.2.7.1"/>
<templateId root="2.16.840.1.113883.10.20.22.2.7.1" extension="2015-08-01"/>
<code code="47519-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="HISTORY OF PROCEDURES"/>
<title>Procedures</title>
<text>
<table>
<thead>
<tr>
<th>Description</th>
<th>Date and Time (Range)</th>
<th>Status</th>
<th>Notes</th>
</tr>
</thead>
<tbody>
<tr ID="Procedure1">
<td ID="ProcedureDesc1">Laparoscopic appendectomy</td>
<td>(03 Feb 2014 09:22am- 03 Feb 2014 11:15am)</td>
<td>Completed</td>
<td ID="ProcedureNote1">
<paragraph>Dr. Physician - 03 Feb 2014</paragraph>
<paragraph>Free-text note about the procedure.</paragraph>
</td>
</tr>
</tbody>
</table>
</text>
<entry typeCode="DRIV">
<!-- Procedures should be used for care that directly changes the patient's physical state.-->
<procedure moodCode="EVN" classCode="PROC">
<templateId root="2.16.840.1.113883.10.20.22.4.14"/>
<id root="64af26d5-88ef-4169-ba16-c6ef16a1824f"/>
<code code="6025007" displayName="Laparoscopic appendectomy"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED-CT">
<originalText>
<reference value="#ProcedureDesc1"/>
</originalText>
</code>
<text>
<reference value="#Procedure1"/>
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="20140203092205-0700"/>
<high value="20140203111514-0700"/>
</effectiveTime>
<!-- Note Activity entry -->
<entryRelationship typeCode="COMP">
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.202" extension="2016-11- 01"/>
<code code="34109-9" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Note">
<translation code="28570-0" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Procedure note"/>
</code>
<text>
<reference value="#ProcedureNote1"/>
</text>
<statusCode code="completed"/>
<!-- Clinically-relevant time of the note -->
<effectiveTime value="20140203"/>
<!-- Author Participation -->
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119"/>
<!-- Time note was actually written -->
<time value="20140204083215-0500"/>
<assignedAuthor>
<id root="20cf14fb-b65c-4c8c-a54d-b0cca834c18c"/>
<name>Dr. Physician</name>
</assignedAuthor>
</author>
<!-- Reference to encounter -->
<entryRelationship typeCode="COMP" inversionInd="true">
<encounter>
<!-- Encounter ID matches an encounter in the Encounters Section -->
<id root="1.2.3.4"/>
</encounter>
</entryRelationship>
</act>
</entryRelationship>
</procedure>
</entry>
</section>
Example 64: Note Activity as Standalone Entry
<section>
<!-- C-CDA Procedures Section -->
<templateId root="2.16.840.1.113883.10.20.22.2.7.1" extension="2014-06-09"/>
<code code="47519-4" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="HISTORY OF PROCEDURES"/>
<title>Procedures</title>
<text>
<list>
<item ID="ProcedureNote1">
<paragraph>Dr. Physician - 03 Feb 2014</paragraph>
<paragraph>Free-text note about procedures which have occurred during this
visit.</paragraph>
</item>
</list>
</text>
<!-- If section were entries required, an additional <entry nullFlavor="NI"> would be
required for a Procedure Activity -->
<!-- Note Activity entry -->
<entry>
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.202" extension="2016-11- 01"/>
<code code="34109-9" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Note">
<translation code="28570-0" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
displayName="Procedure note" />
</code>
<text>
<reference value="#ProcedureNote1" />
</text>
<statusCode code="completed"/>
<!-- Clinically-relevant time of the note -->
<effectiveTime value="20140203" />
<!-- Author Participation -->
<author>
<templateId root="2.16.840.1.113883.10.20.22.4.119" />
<!-- Time note was actually written -->
<time value="20140204083215-0500" />
<assignedAuthor>
<id root="20cf14fb-b65c-4c8c-a54d-b0cca834c18c" />
<name>Dr. Physician</name>
</assignedAuthor>
</author>
<!-- Reference to encounter -->
<entryRelationship typeCode="COMP" inversionInd="true">
<encounter>
<!-- Encounter ID matches an encounter in the Encounters Section -->
<id root="1.2.3.4" />
</encounter>
</entryRelationship>
</act>
</entry>
</section>
Example 65: Rich Text Format (RTF) Example
<section>
<!--... -->
<text>
<list>
<item ID="note1">
<caption>Nursing Note written by Nick Nurse</caption>
<paragraph>Completed rounds; no incident</paragraph>
</item>
</list>
</text>
<!-- Note Activity (extra markup removed to focus on <text>) -->
<entry>
<act>
<code>...</code>
<text mediaType="text/rtf" representation="B64">
<reference value="#note1"/>
</text>
<!--...-->
</act>
</entry>
</section>
In certain situations, clinicians need to record why something was or was not done. C-CDA provides three methods to record this information: