Pharmaceutical Quality (Industry), published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 1.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/uv-dx-pq/ and changes regularly. See the Directory of published versions
Details about manufacturing process validation protocols and results.
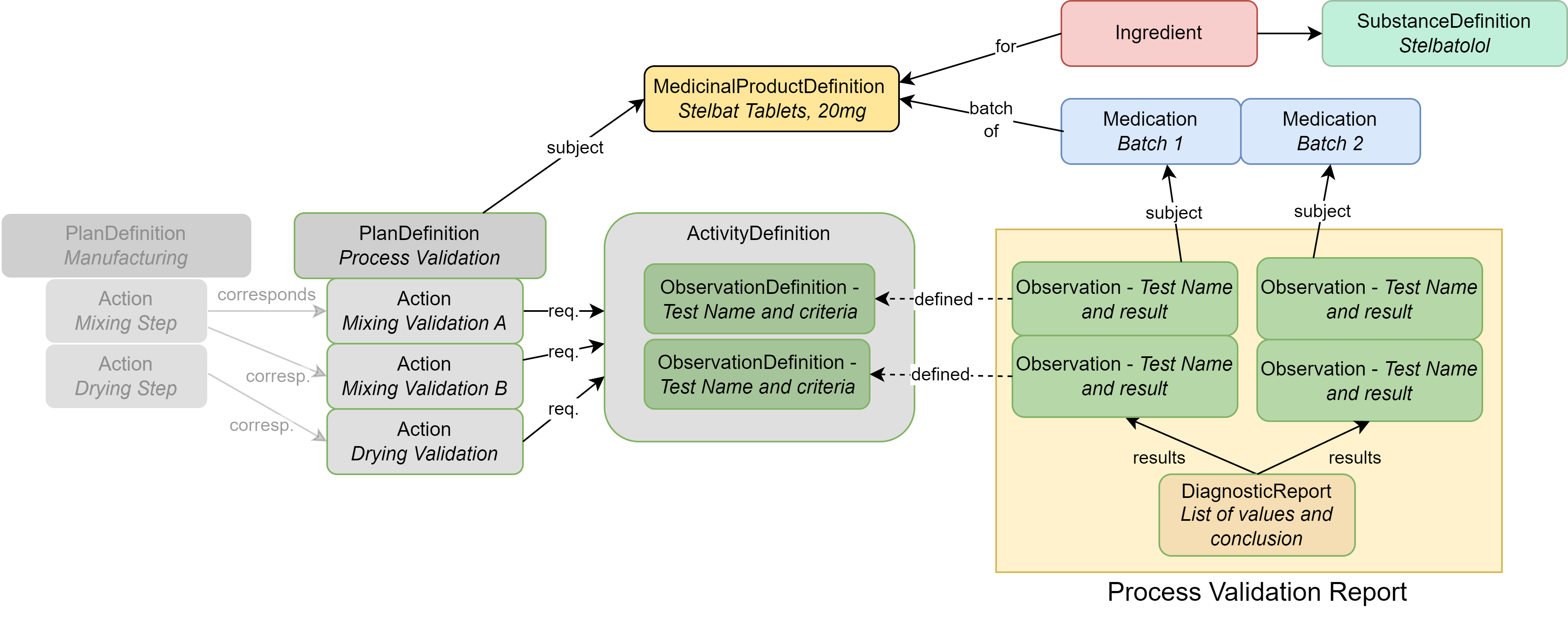 |
| MedicinalProductDefinition | The drug product (Stelbat tablets, 20mg) |
| Ingredient | The active ingredient (stelbatalol) or the ingredients that make up the drug product |
| SubstanceDefinition | Chemical or biological details about substance(s) associated with the ingredient |
| Medication | Describes the batches that underwent testing |
| PlanDefinition | Describes the process validation protocol |
| ObservationDefinition | Each individual test and acceptance criteria; also used to group closely related tests |
| ActivityDefinition | Used to group tests such as by timings |
| Observation | The results of a specific test mentioned in the ObservationDefinition |
| DiagnosticReport | Contains all results as a group and captures conclusions |
| Organization | (not illustrated above): The company/site that performed the testing or manufacturing |
| Procedure | (not illustrated above): Records the actual performance of a manufacturing step, so that measurements can be recorded via Observations (e.g., “hold time”, “bioburden”) |
| Substance | Identifying information about an actual batch of substance (an instance) |
CTD section synthetic source data samples (PDF):
XML and JSON examples of synthetic quality data:
HTML presentation example of synthetic quality data:
Note that HTML examples represent ways to create human-readable output using structured quality data (see section 1.2 Scope).
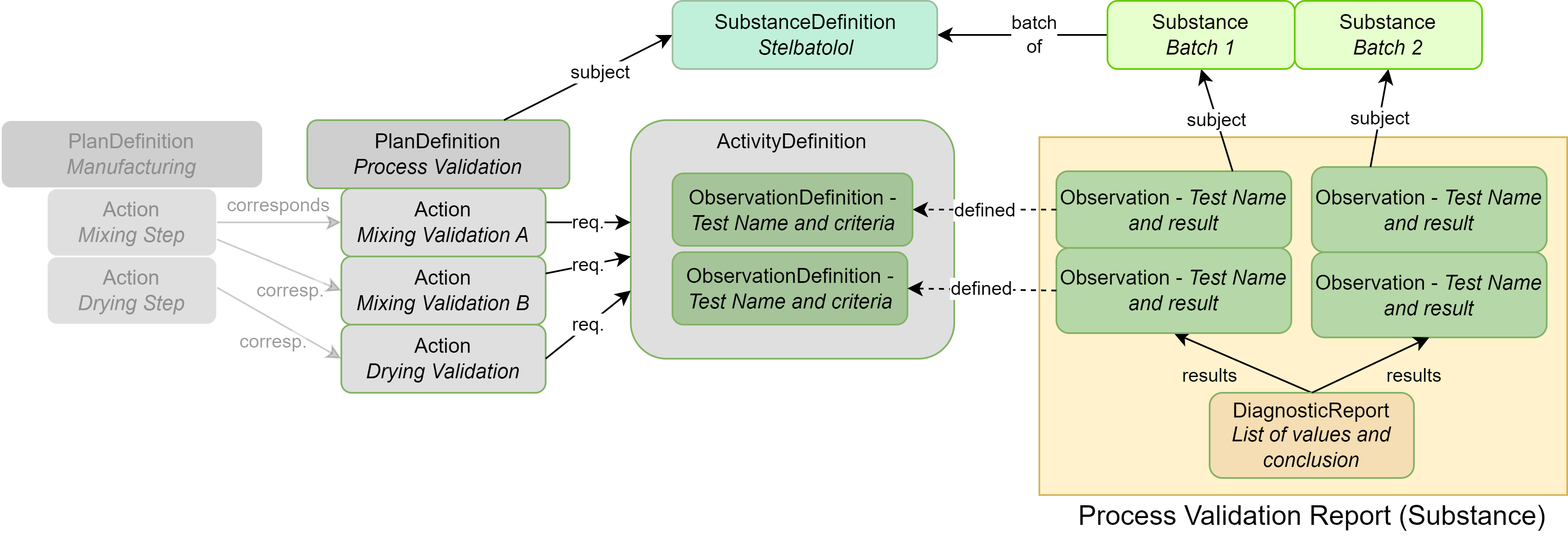 |
| SubstanceDefinition | Chemical or biological details about substance(s) associated with the ingredient |
| Substance | Identifying information about an actual batch of substance (an instance) |
| PlanDefinition | Describes the process validation protocol |
| ObservationDefinition | Each individual test and acceptance criteria; also used to group closely related tests |
| ActivityDefinition | Used to group tests such as by timings |
| Observation | The results of a specific test mentioned in the ObservationDefinition |
| DiagnosticReport | Contains all results as a group and captures conclusions |
| Organization | (not illustrated above): The company/site that performed the testing or manufacturing |
CTD section synthetic source data samples (PDF):
XML and JSON examples of synthetic quality data:
HTML presentation example of synthetic quality data: