Pharmaceutical Quality - Chemistry, Manufacturing and Controls (PQ-CMC) Submissions to FDA, published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 2.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/FHIR-us-pq-cmc-fda/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
The General Information bundle profile provides a mechanism for the industry to submit Module 3 CTD 3.2.S.1 folder content to the FDA. This is a higher level CTD folder. At present this folder’s scope is the drug substance 3.2.S.1.1 - Nomenclature and 3.2.S.1.2 - Structure. Some of the content of these sections are described below –
The domain concepts of Substance Nomenclature and Structure are represented in FHIR in this IG section. Below is a high-level FHIR resource mapping to guide understanding of how the domain concepts are represented using profiles on FHIR resources. Detail study of the profiles and each of the resources will be needed to develop a deeper understanding of this Substance General Information FHIR Profile. Concepts that are key to this domain include the following:
Note: profile computable names (in parenthesis above) map to names in the Profile Map below.
Not presently defined. Content will be added in the future when FDA PQ/CMC FHIR IG starts supporting other scenarios, for example new dosage forms such as liquids, etc.
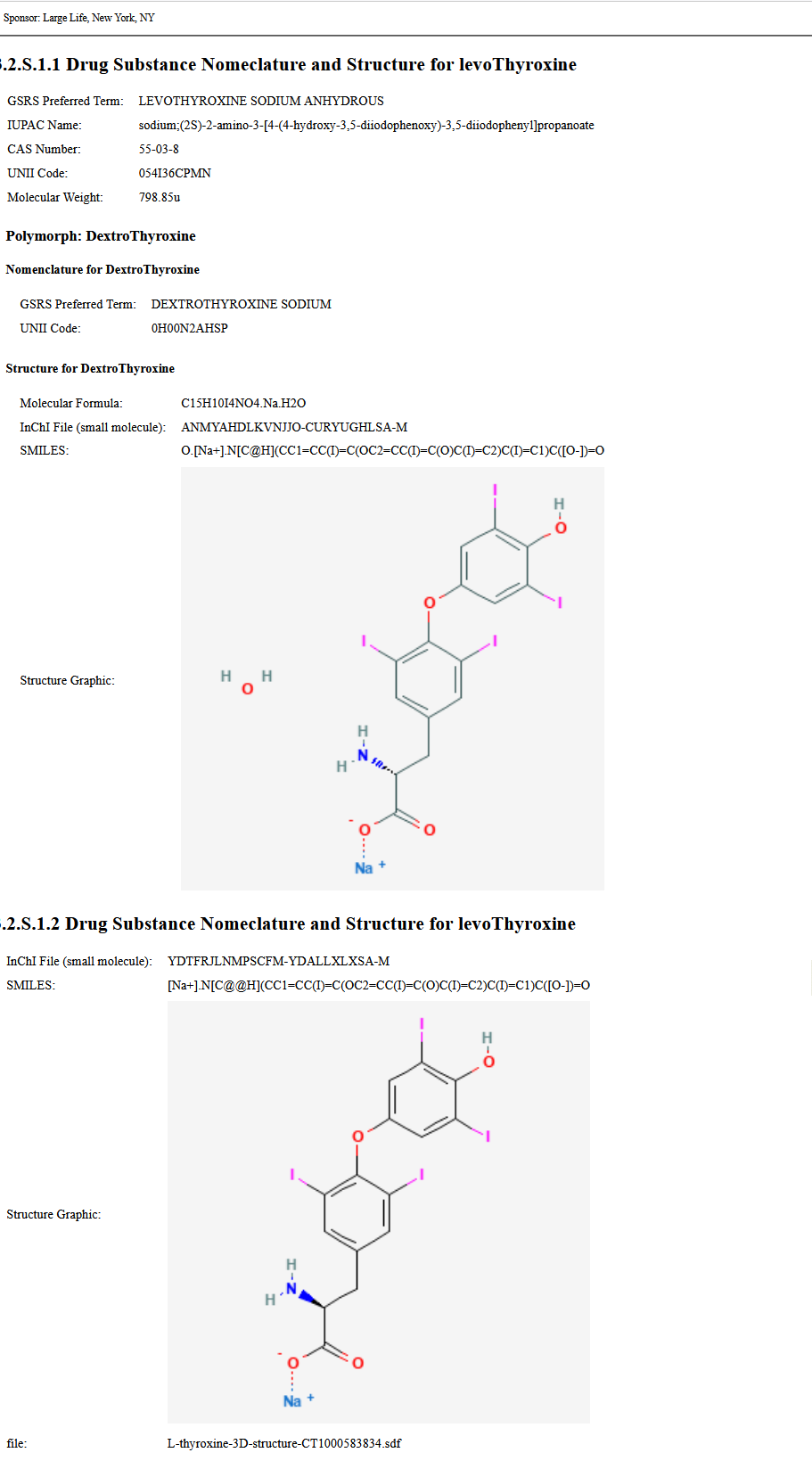
This image demonstrates a Drug Substance with a polymorph displayed with narrative inserted in the composition text element. The XML can be found on the Artifacts page. The XML file with the publisher narrative is on the artifacts page and in the Bundle profile. GeneralInformationBundle
The file includes a SDF file as an attachment. After processing with the narrative tranform available on the Downloads page, clink on the file link at the bottom.