Pharmaceutical Quality - Chemistry, Manufacturing and Controls (PQ-CMC) Submissions to FDA, published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 2.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/FHIR-us-pq-cmc-fda/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
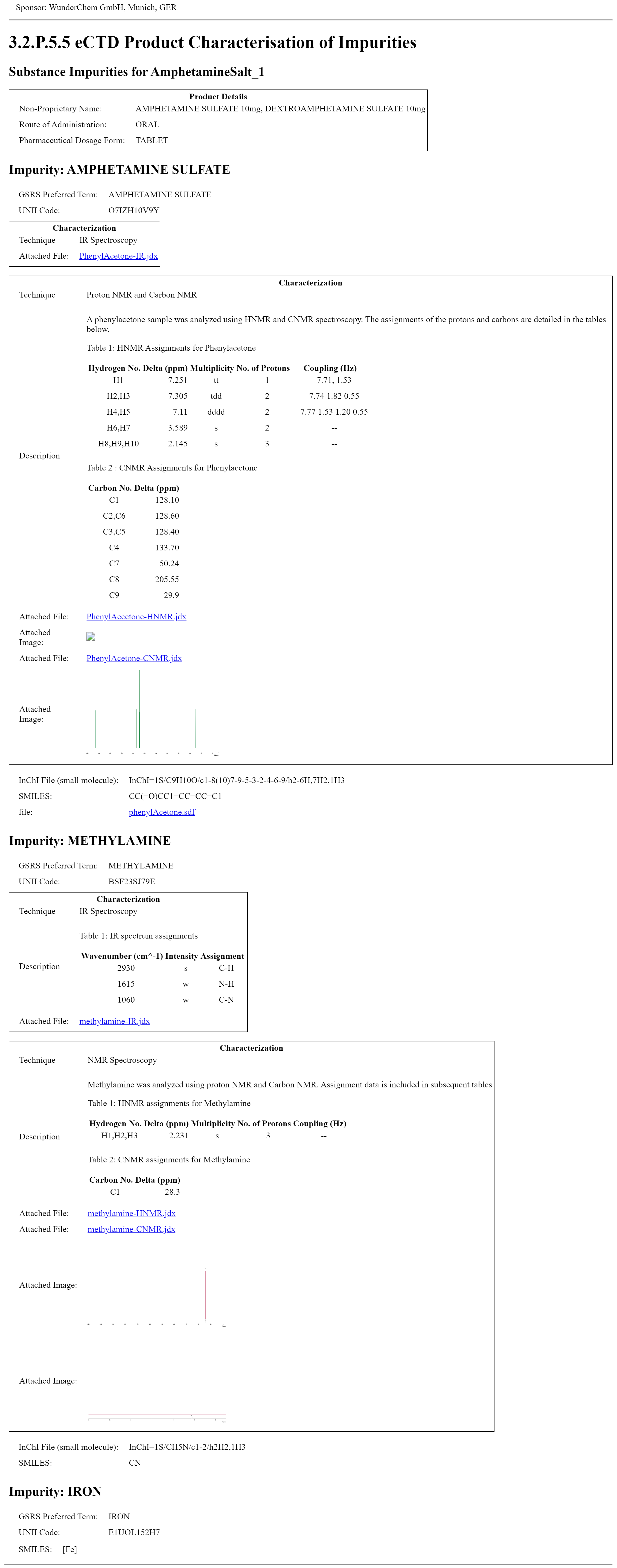
The Product Characterisation of Impurities bundle profile provides a mechanism for the industry to submit Module 3 CTD 3.2.P.5.5 folder content to the FDA. For each impurity found within the drug substance, in addition to identifying the impurity by name, and UNII if known, the content includes pictorial representations of the impurities produced as the result of elucidating the structure or characterization of the impurity through various techniques, including, but not limited to, NMR and mass spectrometry.
Note: profile computable names (in parenthesis above) map to names in the Profile Map below.
Not presently defined. Content will be added in the future when FDA PQ/CMC FHIR IG starts supporting other scenarios, for example new dosage forms such as liquids, etc.
This example demonstrates the impurities contained within a drug product that is a coated tablet that contains one bead type that has two coatings. This image displays the narrative as inserted in the composition text element generated by the narrative transform. The XML can be found on the Artifacts page. The XML file with the publisher narrative is on the artifacts page and in the Bundle profile. CharacterisationImpuritiesProductBundle A second example is CharacterisationImpuritiesBundle.