Pharmaceutical Quality - Chemistry, Manufacturing and Controls (PQ-CMC) Submissions to FDA, published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 2.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/FHIR-us-pq-cmc-fda/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
The Product Batch Formula bundle profile provides a mechanism for the industry to submit Module 3 CTD 3.2.P.3.2 folder content to the FDA. The contents of this section include the amount of material in a specific type of batch, in total and broken down by the components and constituents that constitute the drug product. Please refer to the Domain Overview Section 7.1 of the Description and Composition of Drug Product (3.2.P.3.1) for explanation of Components and Constituents.
Note: profile computable names (in parenthesis above) map to names in the Profile Map below.
The batch formula structure varies at ManufacturedItemDefinition.component. Two XML snippets shown here represent different types of drug products with distinct structures for their ingredients. The first snippet represents a drug product that is a solution with a set of ingredients specified as constituents. This applies to a single layer tablet also. The second snippet represents a layered pill drug product with multiple layers and constituents within each layer.
First XML Snippet: Solution
<type>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C42986"/>
<display value="Solution"/>
</coding>
</type>
<amount>
<value value="53.2"/>
<unit value="kilogram"/>
<system value="http://unitsofmeasure.org"/>
<code value="kg"/>
</amount>
<amount>
<value value="100"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<constituent>
<amount>
<value value="25.42"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="0.0477"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<hasIngredient>
<reference>
<reference value="urn:uuid:1c1a2a23-1fd7-4487-9682-49877f9f9f77"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="20.5"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="0.0385"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<hasIngredient>
<reference>
<reference value="urn:uuid:12cce82f-8595-4860-b7ca-06c9ec0327f2"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="5"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="0.0094"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<hasIngredient>
<reference>
<reference value="urn:uuid:45eb4f05-83d9-4819-bb60-96c7c5ce2b76"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
...
</constituent>
<property>
<type>
<text value="Product Part Identifier"/>
</type>
<valueCodeableConcept>
<text value="solution"/>
</valueCodeableConcept>
</property>
</component>
Second XML Snippet: Layered Pill Drug Product
<component> <!--FIRST COMPONENT -->
<type>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C66831"/>
<display value="Layer"/>
</coding>
</type>
<amount>
<value value="925.97"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="38.55"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<constituent>
<amount>
<value value="400"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="43.1"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203881"/>
<display value="Active core/granulate"/>
</coding>
<text value="Purple Layer"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:d4713a90-38d1-49e5-a977-78c63e518562"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="524.01"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="56.59"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203883"/>
<display value="Intragranular"/>
</coding>
<text value="Purple Layer"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:9973ba9a-b257-425a-8fb9-6dcfe379ca08"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="203.71"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="22"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203882"/>
<display value="Extragranular"/>
</coding>
<text value="Purple Layer"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:4e394a0c-4b25-492e-aac7-0faf121ffc4f"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="305.57"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="33"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203882"/>
<display value="Extragranular"/>
</coding>
<text value="Purple Layer"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:95648837-ec81-42d9-970c-eac390f2f604"/>
</reference>
</hasIngredient>
</constituent>
<property>
<type>
<text value="Product Part Identifier"/>
</type>
<valueCodeableConcept>
<text value="Purple Layer"/>
</valueCodeableConcept>
</property>
</component>
<component> <!--SECOND COMPONENT -->
<type>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C66831"/>
<display value="Layer"/>
</coding>
</type>
<amount>
<value value="1476.03"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="61.45"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<constituent>
<amount>
<value value="600.01"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="40.65"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203881"/>
<display value="Active core/granulate"/>
</coding>
<text value="Example1Drug_LayerB"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:fbd464cc-2511-4a3a-b058-3cec4fffd14f"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="876.02"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="59.35"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203883"/>
<display value="Intragranular"/>
</coding>
<text value="Example1Drug_LayerB"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:9973ba9a-b257-425a-8fb9-6dcfe379ca08"/>
</reference>
</hasIngredient>
</constituent>
<constituent>
<amount>
<value value="369.01"/>
<unit value="gram"/>
<system value="http://unitsofmeasure.org"/>
<code value="g"/>
</amount>
<amount>
<value value="25"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203882"/>
<display value="Extragranular"/>
</coding>
<text value="Example1Drug_LayerB"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:95648837-ec81-42d9-970c-eac390f2f604"/>
</reference>
</hasIngredient>
</constituent>
<property>
<type>
<text value="Product Part Identifier"/>
</type>
<valueCodeableConcept>
<text value="Example1Drug_LayerB"/>
</valueCodeableConcept>
</property>
</component>
Key Differences in Structure:
In summary, the second XML snippet represents a more complex, layered pill structure with additional metadata about the location and identity of each layer and its constituents. The first snippet is a simpler structure, representing a solution with ingredients described at a higher level of abstraction.
This extension is used to capture the overage amount and reason. Overage refers to an intentional extra quantity of an active ingredient or excipient added to a drug product batch, beyond the amount required to achieve the labeled dosage. The overage compensates for potential losses during manufacturing, such as ingredient degradation, evaporation, or losses in processing. It is essentially a "buffer" to ensure that the final product meets the required strength or concentration.
Reasons for Overages
There are several reasons why overages are used in drug manufacturing, including:
Example of Overages in a Drug Product Batch Record
As an example of a tablet manufacturing batch record for a drug product where the active ingredient (e.g., "Acetaminophen") is required to be 500 mg per tablet. To account for potential losses and ensure potency, an overage of 2% is added.
Batch Formula Overage Example:
Thus, the batch formula for the Acetaminophen tablet would include 510 mg of the active ingredient per tablet to ensure that after manufacturing losses, each tablet contains the correct 500 mg of the active ingredient.
Here’s how this would be reflected in the ManufacturedItemDefinition.component.constituent:
<constituent>
<extension url="http://hl7.org/fhir/us/pq-cmc-fda/StructureDefinition/pq-overage-extension">
<extension url="percent">
<valueDecimal value="0.02"/>
</extension>
<extension url="justification">
<valueMarkdown value="Manufacturing losses, degradation over time"/>
</extension>
</extension>
<amount>
<value value="510"/>
<unit value="milligram"/>
<system value="http://unitsofmeasure.org"/>
<code value="mg"/>
</amount>
<amount>
<value value="35"/>
<unit value="percent"/>
<system value="http://unitsofmeasure.org"/>
<code value="%"/>
</amount>
<location>
<coding>
<system value="http://ncicb.nci.nih.gov/xml/owl/EVS/Thesaurus.owl"/>
<code value="C203882"/>
<display value="Extragranular"/>
</coding>
<text value="ER Layer"/>
</location>
<hasIngredient>
<reference>
<reference value="urn:uuid:85642235-ec81-42d9-970c-eac390f2f604"/> <!--REFERENCE TO ACETAMINOPHEN -->
</reference>
</hasIngredient>
</constituent>
In this example:
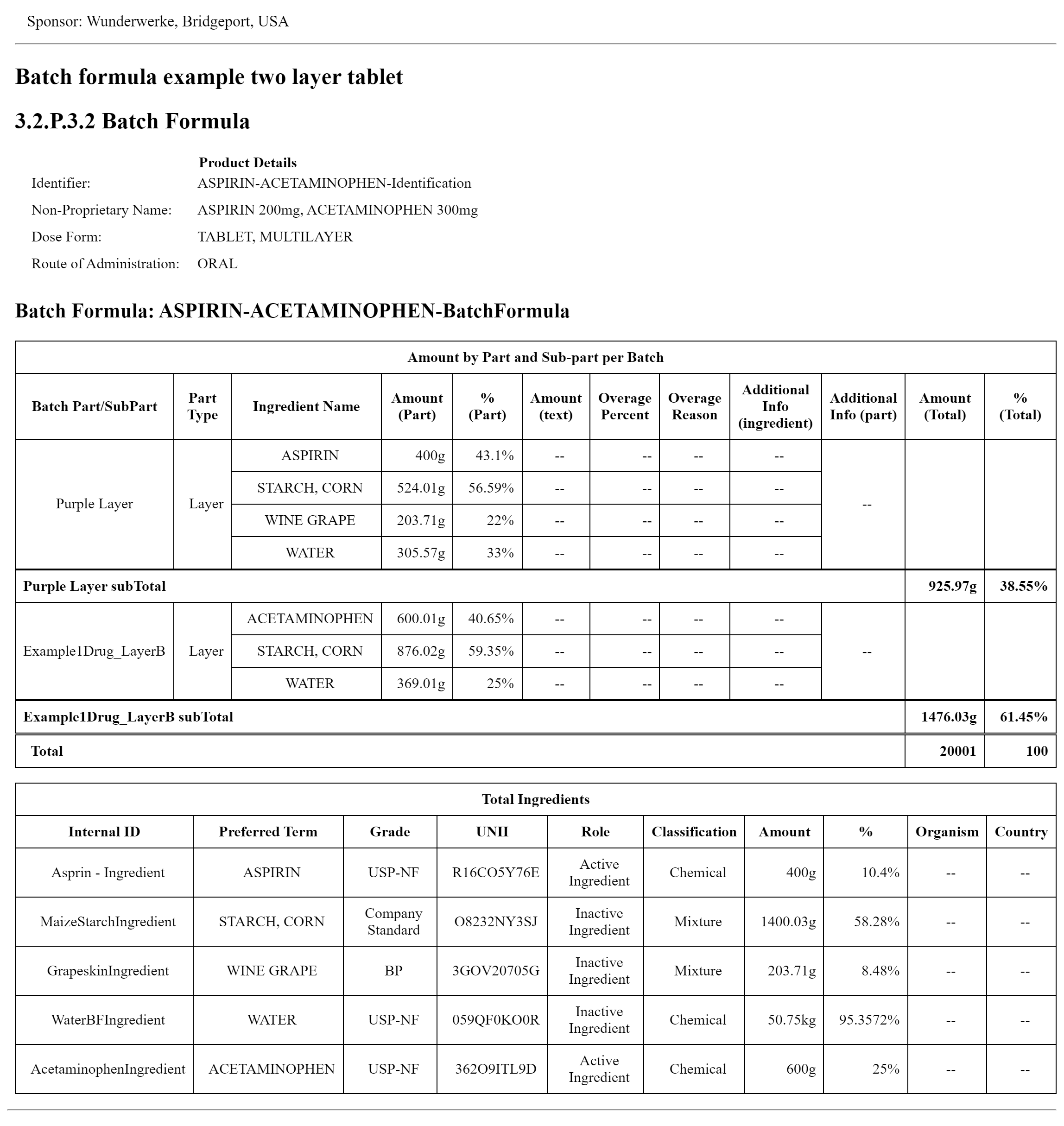
This example demonstrates the batch formula for a commercial batch of a drug product. This image displays the narrative as inserted in the composition text element generated by the narrative transform. The XML can be found on the Artifacts page. The XML file with the publisher narrative is on the artifacts page and in the Bundle profile. BatchFormulaBundle2Layer
Another batch formula example is BatchFormulaBundle