Genomics Reporting Implementation Guide, published by HL7 International / Clinical Genomics. This guide is not an authorized publication; it is the continuous build for version 4.0.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/genomics-reporting/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
This section covers additional guidelines related to genetic assessments related to the histocompatibility and immunogenetics, including matching of donor and recipient tissue for donation (e.g., bone marrow, organ, and other tissue donations) and cellular therapy. In particular, it covers additional guidance related to genotyping reports of HLA (Human Leukocyte Antigens).
While not specifically profiled in this version of the IG, some additional constraints will typically apply to somatic profiles. The Patient resource is often mandatory as transplant genomics is not relevant for environmental samples. Exceptions would include when de-identified samples are sent to a typing laboratory, and patient information is stored separately at an organization.
Human leukocyte antigen (HLA) genotyping is fundamental for research and clinical practice in immunogenetics and histocompatibility. Pointers to external locations referring to registered methods, raw NGS reads, and reference standards can be conveyed in this profile.
The structure of the HLA typing report in this profile attempts to follow the principles outlined in the Minimum Information for Reporting Next-generation sequence Genotyping (MIRING). These principles were identified through a series of meetings with an international group of stakeholders in the application of Next Generation Sequencing (NGS) technology for genotyping the HLA and KIR genes as well as other immune-related genes (http://igdawg.org/ngs.html). MIRING describes eight principles, described in detail in Human Immunology. 2015 Dec; 76(12):954-962. These include detailed metadata about:
These principles were implemented in a technical specification by extending an existing XML based format for exchanging histocompatibility and immunogenetic genotyping data called Histoimmunogenetics Markup Language (HML) to include results from NGS methodologies. The resulting schema may be found here. The National Marrow Donor Program (NMDP)/Be The Match uses this format for reporting HLA genotyping from potential donors and for patients needing stem cell transplants.
Both MIRING and HML were used to inform mapping data elements to FHIR resources profiled in this IG, such as Specimen, MolecularSequence, Genotype Observation, Haplotype Observation, and DiagnosticReport. Examples are found at the end of this page.
Once HLA is typed, perhaps using targeted probes, exon sequencing, or full gene sequencing, the results are analyzed and assigned to one or more of the IMGT/HLA gene alleles using the HLA nomenclature. While the actual results may be a haplotype of exons, the results are assigned to an allele from a known list. This allows results from different HLA genotyping methodologies to be compared, for example for donor/recipient transplant matching. However, reducing the results to HLA nomenclature may introduce ambiguity in the actual results. For example, an allele may be reported at only a two-field protein level (e.g., HLA-B*57:01) which represents any of over forty DNA variants, while the actual data may represent a single full gene (e.g., HLA-B*57:01:01:05). The nomenclature may also over represent the quality of the data. For example, when a full gene allele name is assigned to the results, it is unknown how the data was captured (probes, exon-only sequence, or full gene sequencing). Methodology is captured separately in the report. Also, this system cannot easily capture novel alleles as it represents a closed-world of possible alleles. Earlier scientific articles and data may reference HLA serotypes (e.g., HLA-B57), recognizable by the absence of an asterisk in the name, which does not capture any molecular information. The IMGT/HLA allele database is updated every three months to account for the discovery of new alleles, and the deletion or renaming of old alleles. This is why it is important to include the IMGT/HLA version when the HLA allele is assigned.
In a simple case, the Genomic Report (DiagnosticReport) refers to a Genotype Observation for each HLA gene. The HLA alleles found in the genotype are typically assigned to alleles found in the Immuno Polymorphism Database (IPD) through the international ImMunoGeneTics (IMGT) project (found here) and preferably expressed as a Genotype List String (GLString) using a GLString Code.
If it is necessary to report the molecular sequence that was used to derive the Genotype Observation, one or more MolecularSequence resources can be used. This is shown in Fig 1.
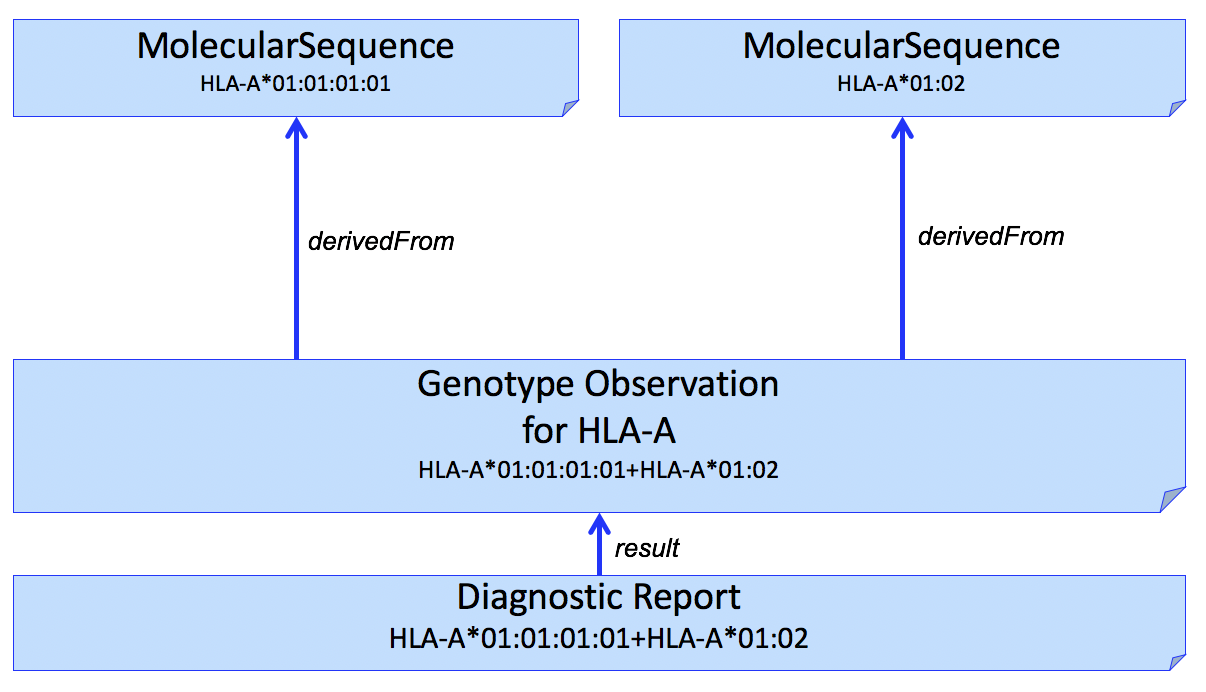
Figure 1
It is often desirable to identify sequence data that was used to assert or assign individual HLA alleles. This can be done by adding another Observation to capture this, shown in Figure 2.
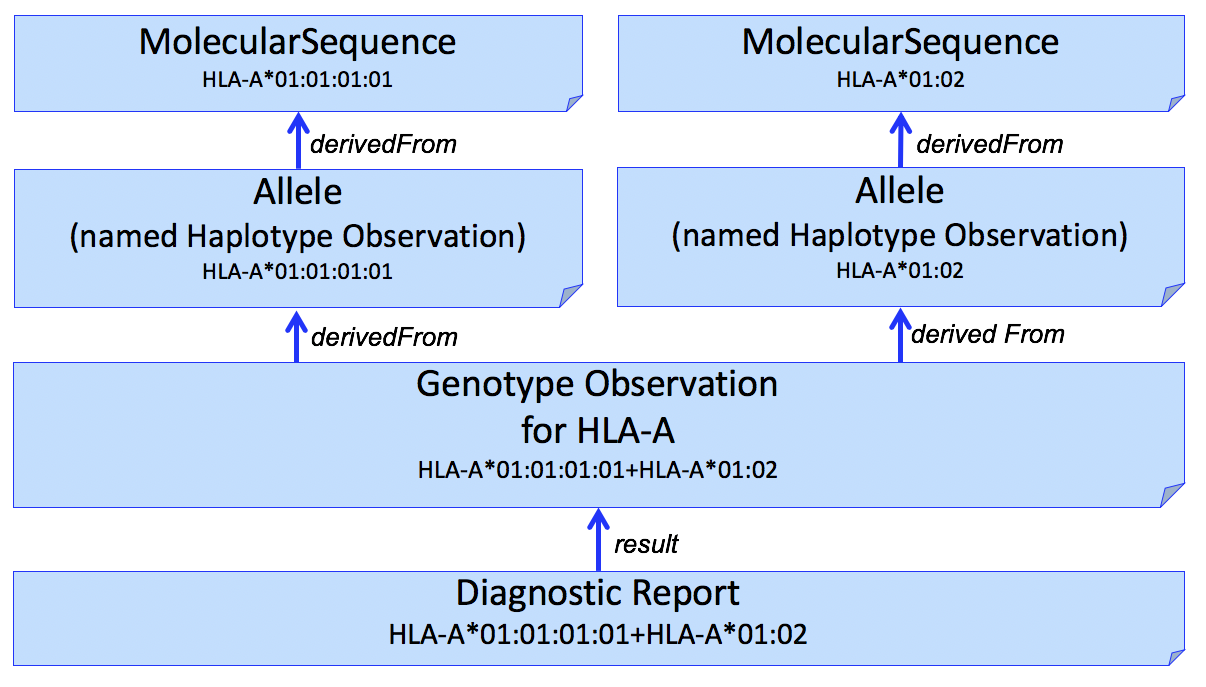
Figure 2
The example in this figure is simplistic and the actual results often includes multiple sequences (e.g., exons 2 and 3). Allele assignment is a complex process and may result in ambiguous results (see Reporting Ambiguity below).
We use the Haplotype Observation for Allele assignment. In domains other than HLA (e.g., Pharmacogenomics), it is common to consider gene-level alleles as named haplotypes of a set of smaller variants (Variation Modelling Collaboration Data Model and Specification). This is not how the HLA community considers Haplotype, which is typically used to describe a set of gene-level alleles from different HLA genes that are found on the same DNA molecule. To be able to represent this latter description, this IG allows the representation "haplotypes of haplotypes." (example not shown)
This pattern can be extended to multiple HLA genes in a single report. An example for HLA-A, HLA-B, and HLA-C is shown in Figure 3.
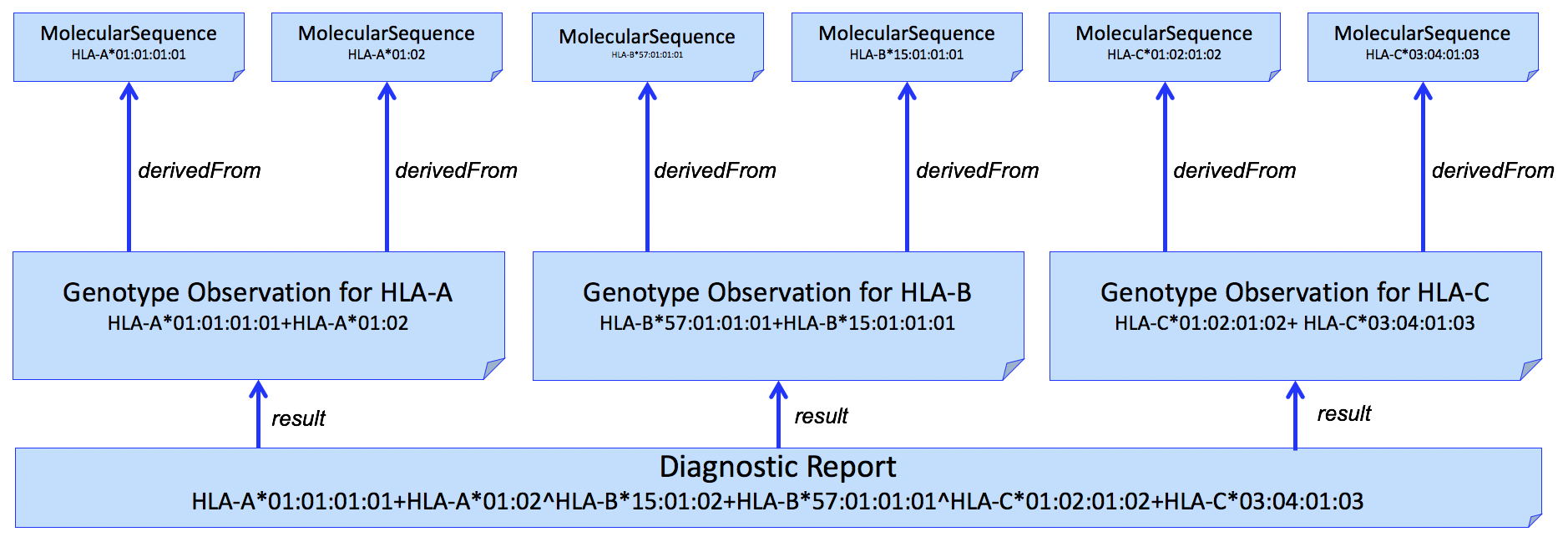
Figure 3
And adding individual alleles...
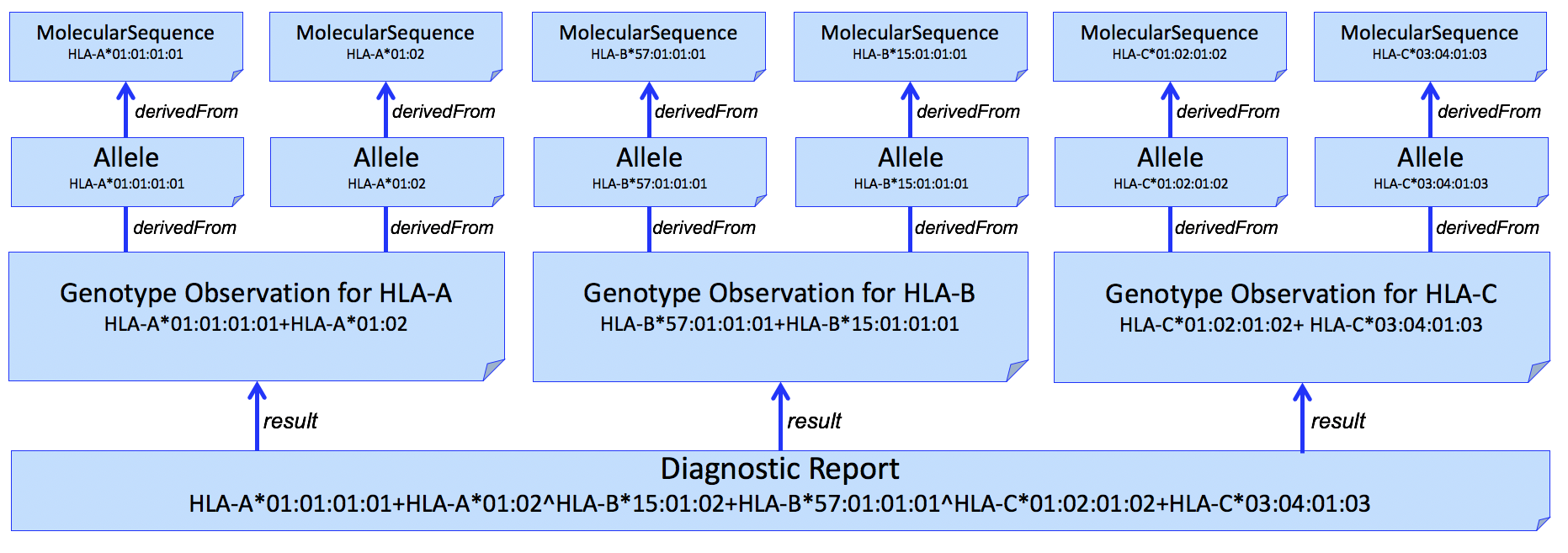
Figure 4
HLA allele assignment is often ambiguous. Genotype ambiguity results from an inability to establish chromosomal phase between identified polymorphisms. Allele ambiguity results when the polymorphisms that distinguish alleles fall outside of the regions assessed by the genotyping system.
Allele ambiguity is often captured using NMDP Multiple Allele Codes. Because of limitations of this system, including introducing further ambiguity into the report, the GL String is preferred. This format uses a hierarchical set of operators to describe the relationships between alleles, lists of possible alleles, phased alleles, genotypes, lists of possible genotypes, and multilocus unphased genotypes, without losing typing information or increasing typing ambiguity. To use this format, the GL String Code system can be used, which embeds this GL String into a format containing the gene system, and version of the nomenclature used within the GL String.
For example, this valueCodeableConcept would be used to describe a genotype for HLA-B, based on the 3.31.0 release of the IMGT-IPD/HLA database, that is ambiguous for one of the alleles in the genotype (HLA-B*07:02:01:01/HLA-B*07:02:01:03), and unambiguous for the other (HLA-B*13:02:01:01).
<valueCodeableConcept>
<coding>
<system value="http://glstring.org"/>
<code value="hla#3.31.0#HLA-B*07:02:01:01/HLA-B*07:02:01:03+HLA-B*13:02:01:01"/>
</coding>
</valueCodeableConcept>
The Observations used in this IG require specific codes. For example, the Genotype Observation requires a code.coding using a LOINC code that represents "Genotype display name". To indicate that this genotype is for HLA-A, we use the component for Gene Studied. Together this code.coding and the component indicate that this Observation is a Genotype for HLA-A. This 'post-coordination' of the Observation code allows for reporting for any genotype and gene name combination.
<code>
<coding>
<system value="http://loinc.org"/>
<code value="84413-4"/>
<display value="Genotype display name"/>
</coding>
</code>
...
<component>
<code>
<coding>
<system value="http://loinc.org"/>
<code value="48018-6"/>
<display value="Gene studied [ID]"/>
</coding>
</code>
<valueCodeableConcept>
<coding>
<system value="http://www.genenames.org"/>
<code value="HGNC:4931"/>
<display value="HLA-A"/>
</coding>
</valueCodeableConcept>
</component>
Often a lab will use another LOINC code to represent this, or use a local code system. This can be included as an additional coding. For example, at the time of this writing, there are ten different LOINC codes related to typing of HLA-A. As long as the alternative coding is conceptual the same or is subsumed by the other, an additional code.coding can be included if the lab or healthcare system uses it in their current system. In the following example, the LOINC code 13298-5 representing "HLA-A [Type]" is added as an additional code.coding that is subsumed by the more general "Genotype Display Name"".
<code>
<coding>
<system value="http://loinc.org"/>
<code value="84413-4"/>
<display value="Genotype display name"/>
</coding>
<coding>
<system value="http://loinc.org"/>
<code value="13298-5"/>
<display value="HLA-A [Type]"/>
</coding>
</code>
...
<component>
<code>
<coding>
<system value="http://loinc.org"/>
<code value="48018-6"/>
<display value="Gene studied [ID]"/>
</coding>
</code>
<valueCodeableConcept>
<coding>
<system value="http://www.genenames.org"/>
<code value="HGNC:4931"/>
<display value="HLA-A"/>
</coding>
</valueCodeableConcept>
</component>
TBD: Considerations for reporting Killer-cell immunoglobin like receptor (KIR) genes.