xShare Project IPS+
0.1.0 - qa-preview
150
xShare Project IPS+
0.1.0 - qa-preview
150
xShare Project IPS+, published by xShare Project. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/hl7-eu/xshare-ips-plus/ and changes regularly. See the Directory of published versions
The technical domain describes the technical stack and the infrastructure required to support the use cases for research of the IPS+ described in this IG.
This includes:
The overarching scope of the xShare IPS+ FHIR Implementation Guide is to provide implementers with additional support for leveraging the HL7 IPS and the supporting documents available within this IG for clinical research and population health. It further builds on the HL7 International Patient Summary Implementation Guide. Although the IPS dataset is a “minimal, non-exhaustive set of data elements required for the international patient summary”, it forms a good basis for use in research and public health. In order to be of use there, a number of recommended and optional categories will be considered as required.
The role of the core harmonized terminology, valuesets and codelist alignment, and the model-to-model mapping included in this scope –FHIR to CDISC mapping of IPS profiles, data element item to item map.
The content available for download includes:
These tools are envisioned to aid in providing semantic interoperability across the FHIR data model with supporting terminologies and the CDISC SDTM data model including the CDISC terminology curated by the NIH NCI EVS Terminology services.
The harmonized terminology, valuesets and codelists can be downloaded and used by implementers or end users to create a standardized database for IPS+ data. Such database can either be integrated in the implementers’ compatible system or used for data mapping through APIs/Queries as detailed in:
The list of specific IPS+ data elements that are specific for the business use cases are represented in the annex II of Analysis of business use cases for use of European EHRxF HIDs in clinical research.
This section outlines how the IPS+ specification could be incorporated within the use cases presented in the application section. The work flow of each use case is described, including the role of the IPS+. It is assumed that the IPS+ data is either held in regional or national repositories, or is retrieved on a patient specific basis by the patient using the xShare yellow button. In practice other IPS+ data flows might be implemented.
The use cases described in the following paragraphs rely on the base setup as described in this simplified figure.
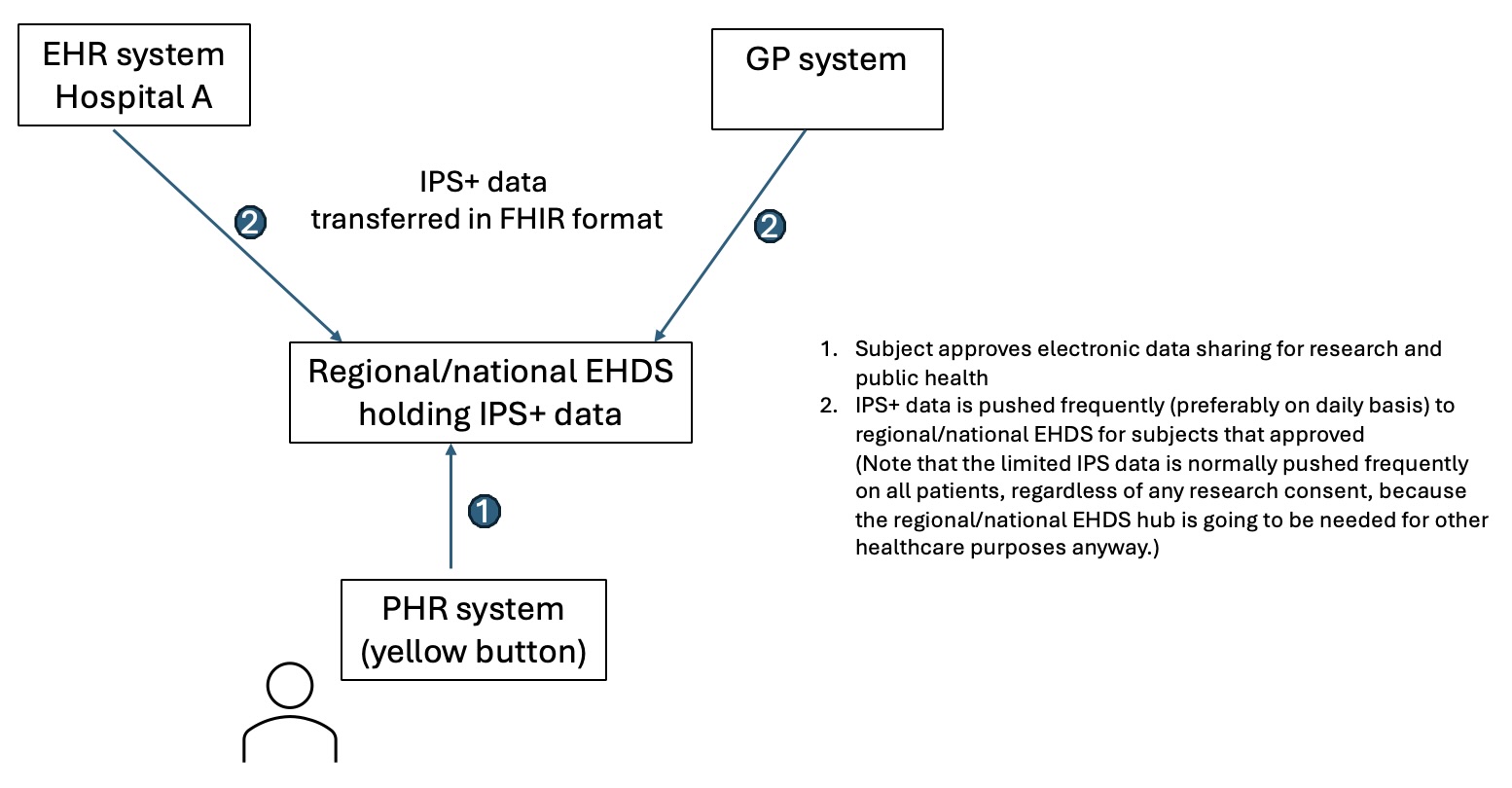
The capability to utilise the IPS+ for study feasibility relies upon a sequence of adoption and implementation decisions within a country, summarised as workflow steps below.
A simplified flow noting the environments used is described in the following figure:
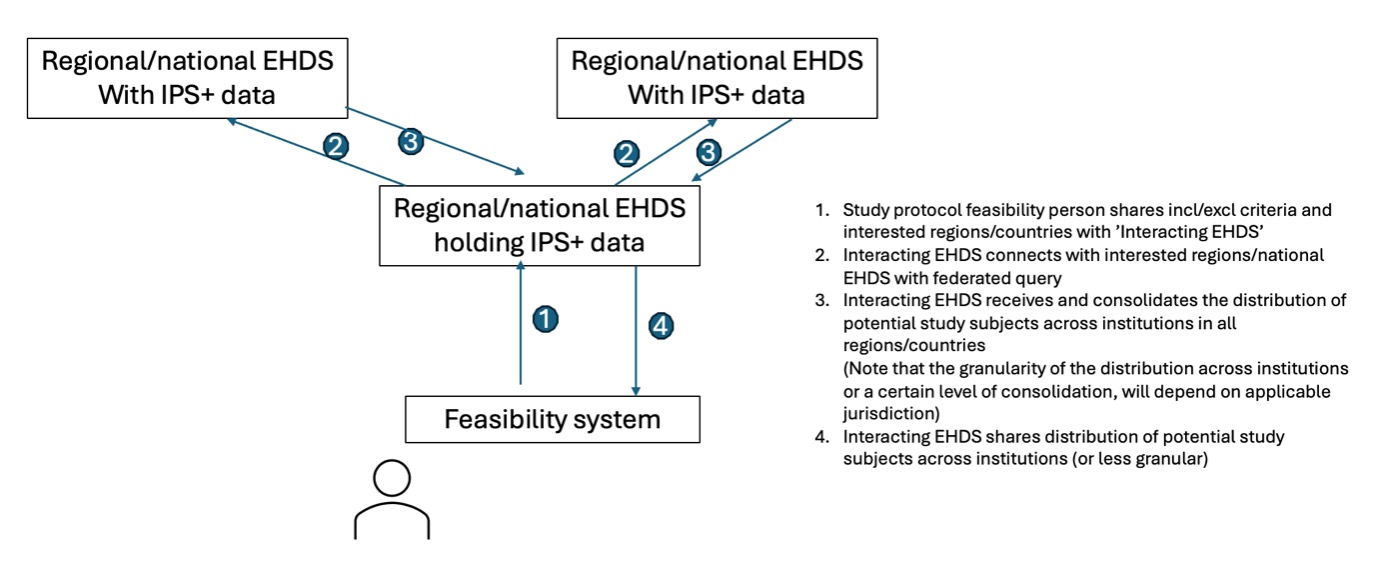
Data sharing for individuals requires the existence of a PHR app connected to the national/regional infrastructure. Health data needed for the pre-screening tool must be available in the IPS+ and able to be exchanged through an interoperability system — the xShare Yellow Button. IPS+ data and national/regional infrastructure's data are standardized and mapped between the IPS and clinical trial system using the standardized value sets and code lists available in the downloads section. It pre-exists in a database either integrated to the pre-screening tool or accessible to recognized third parties through API calls.
Patients and healthy volunteers have access to a PHR app to access their health data, and in this app they can assess their eligibility for a clinical trial. They are interested and want to know if they can be included in a clinical trial, in one of the hospitals near to their home.
PHR and participant workflow steps
A simplified flow noting the environments used is described in the following figure:
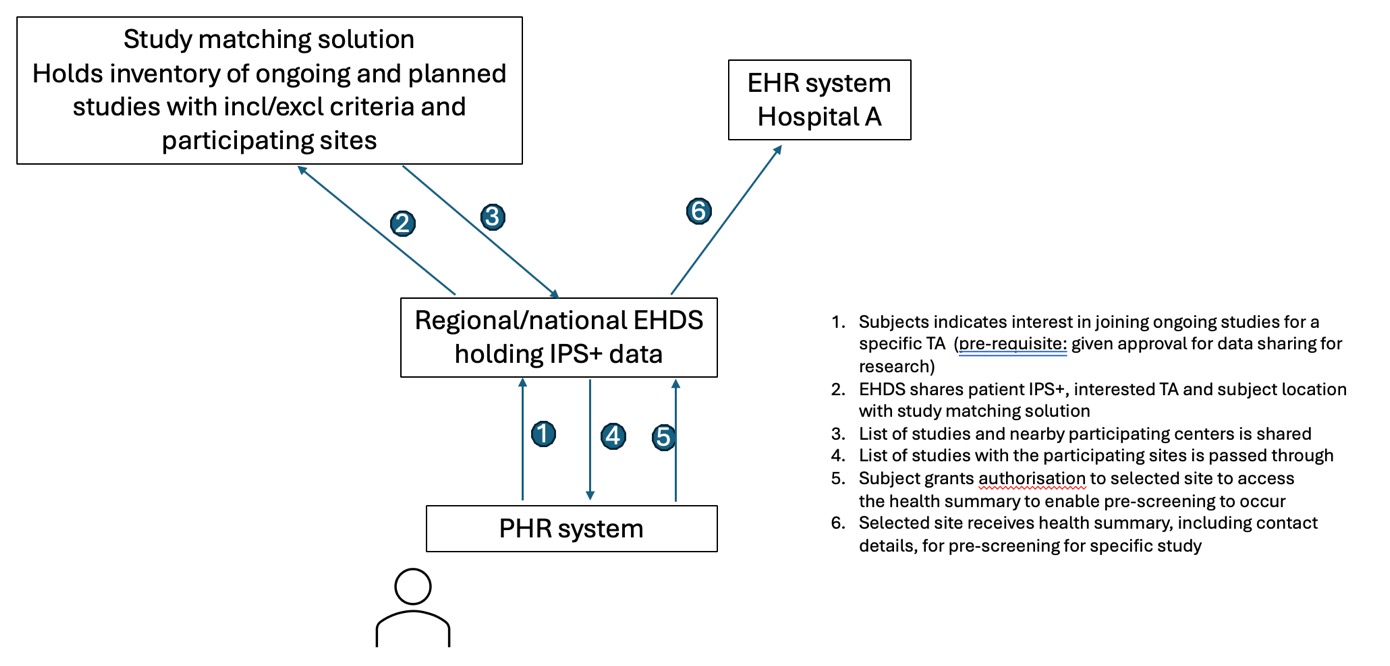
The consenting patient enrolled in the study is included by the investigative team of the centre. The study allows eCRF automatic filling from existing health data. The patient has access to their PHR app containing a section allowing IPS+ data sharing for clinical research. The patient agrees to the automatic health data filling of the eCRF in the context of this study.
A simplified flow noting the environments used in view of a RWE study is described in the following figure:
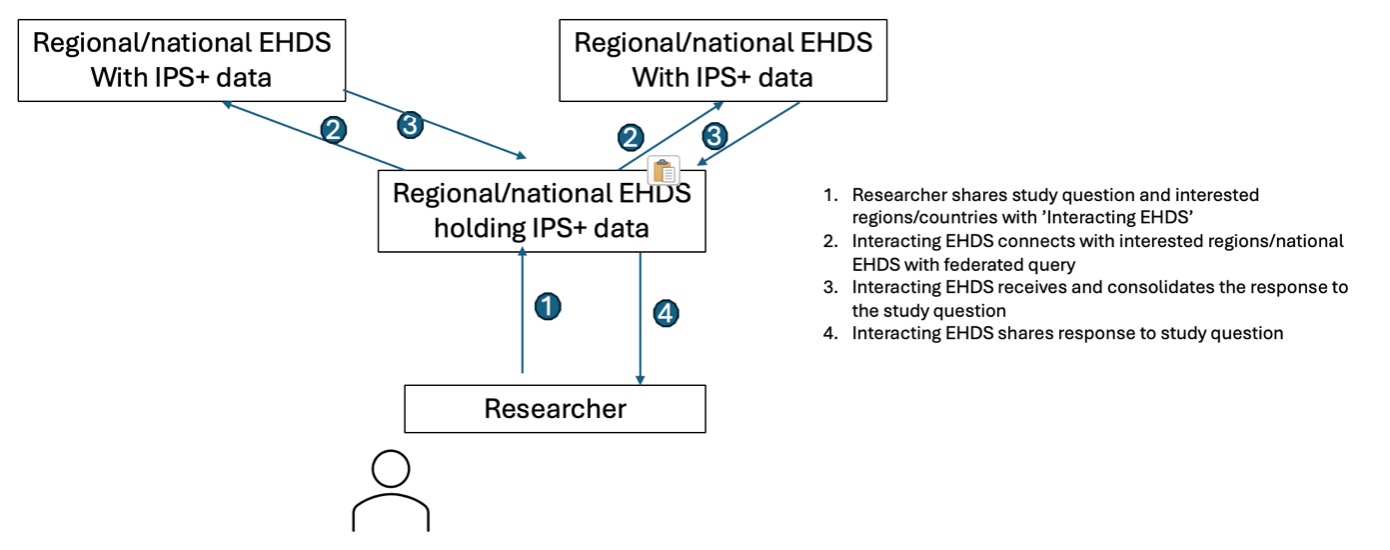
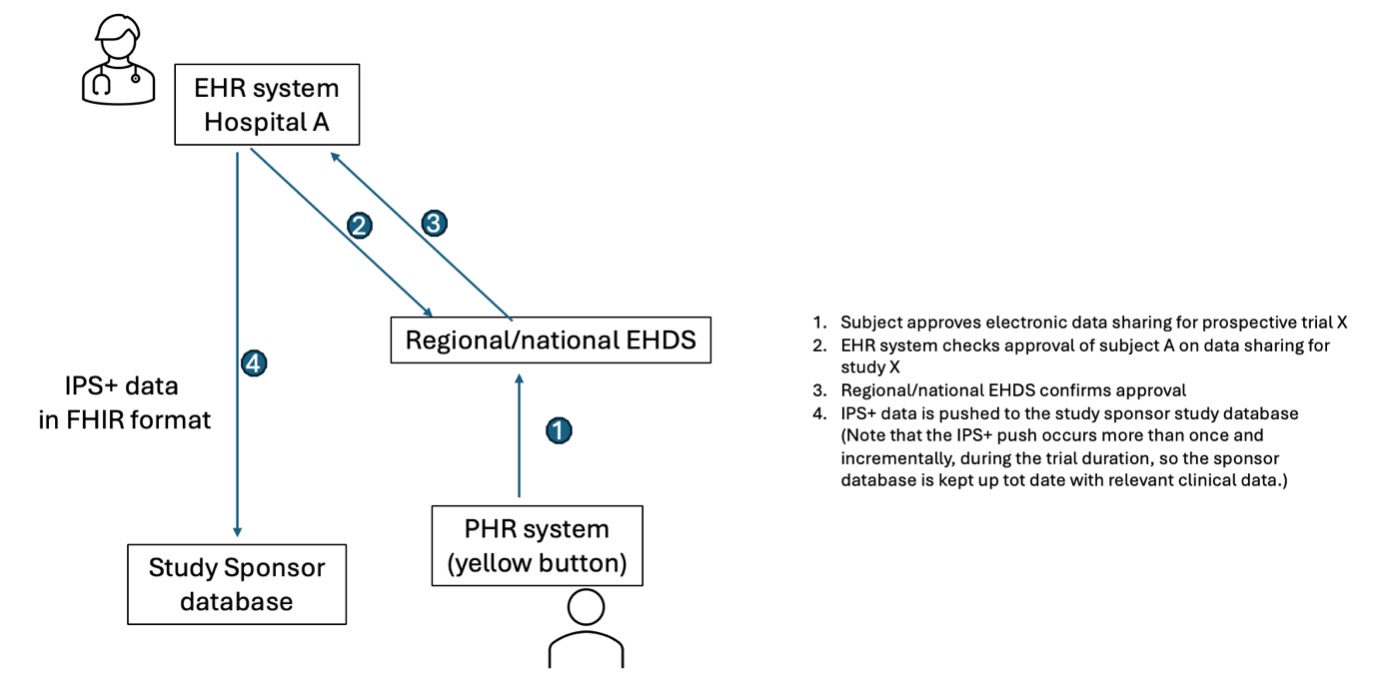
A simplified flow noting the environments used in view of a prospective study is described in the following figure.
Note that step 4 might require a mapping solution between the IPS+ data pushed from the site and the study database with the sponsor.
It should be noted that the clinical study protocol is likely to contain data elements beyond those defined in the IPS+.
The data from the IPS+ will therefore not result in the entire filling of the eCRF.
This workflow therefore provides only part of the study dataset, but is considered valuable because these are frequently copied across from the patient's health record.
If, for example, an examination or visit unrelated to the study occurs while a patient is participating and results in an update to the patient’s medication list, or in an adverse event, the updated IPS+ can be sent to the study centre and data updated in the eCRF.
If a patient changes their mind and no longer wishes their IPS+ data to be used in the study, they can cease forwarding downloads or cancel their authorisation.
(Because clinical trial data needs to be retained long-term as evidence of the trial centre and sponsor duty of care, historically transferred data will not normally be deleted.)
The xShare Yellow Button utilises an enhanced data subject right under the GDPR, by enabling a patient to access a complete copy of their European EHRxF data in a computable, standardised form, utilising the same standards that are used to exchange this information between healthcare systems. The IPS+ is proposed as the representation of this content.
The EHDS Regulation permits an individual to forward their exchange format information to any other parties, for example to obtain a second clinical opinion or to participate in research.
The trial centre should publish its data protection policy and notices, so that the patient is well aware of the GDPR-compliant terms and safeguards that will be applied to any data they provide to the trial centre they have chosen.
The act of forwarding the information effectively provides their informed consent for the recipient to legally hold their personal European EHRxF data according to those published terms and safeguards.
If the patient passes prescreening, screening, and then becomes enrolled in the clinical study, the trial centre and sponsor will then need to retain their copies of the patient’s health information on a long-term basis, as their record of having performed a duty of care to the patient.
This is usually the GDPR legal basis of legitimate interest in industry clinical studies.
This is why, if a patient withdraws from a clinical study, their historic data will not be deleted.
It will be a matter for the trial centre, in its published notices, to make clear what right of deletion a patient may exercise at the pre-screening stage when no duty of care has yet been provided.
The xShare Yellow Button workflows additionally need to be secure, normally to the information security standards that the national health system would utilise for its own communications between healthcare providers or between a healthcare provider and a patient.
Since this representation will utilise HL7 FHIR, it is possible that its information security and access control standards will be used:
The information security measures needed for the data flows that utilise the IPS+ specification are beyond the scope of this implementation guide.
The EHDS Regulation places an obligation on EHR systems to document the consistency, accuracy and completeness of data within the scope of the European EHRxF.
It is not clear from the Regulation how this quality information is to be handled, provided, or communicated whenever an extract is generated and communicated.
This differs from the data quality and utility label to be applied to datasets for secondary use, when the (optional) label is to be included within the data set catalogue.
The EHDS Regulation sets no minimum data quality standard for primary use (but only that it is documented by EHR vendors) and makes no provision for any efforts that should be undertaken by Member States, health systems or EHR vendors to assure good data quality or to improve poor data quality.
This means that the IPS+ data will be obtained “as is”.
If the clinical research use cases have minimum data quality standards — for example, before data is transferred into a study database — then these data quality assessments need to be undertaken by the party orchestrating the data transfer.
Relevant information for research and secondary use of data:
Additional references that may be helpful, however, were not specifically designed targeting secondary uses of data are: