xShare Project IPS+
0.1.0 - qa-preview
150
xShare Project IPS+
0.1.0 - qa-preview
150
xShare Project IPS+, published by xShare Project. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/hl7-eu/xshare-ips-plus/ and changes regularly. See the Directory of published versions
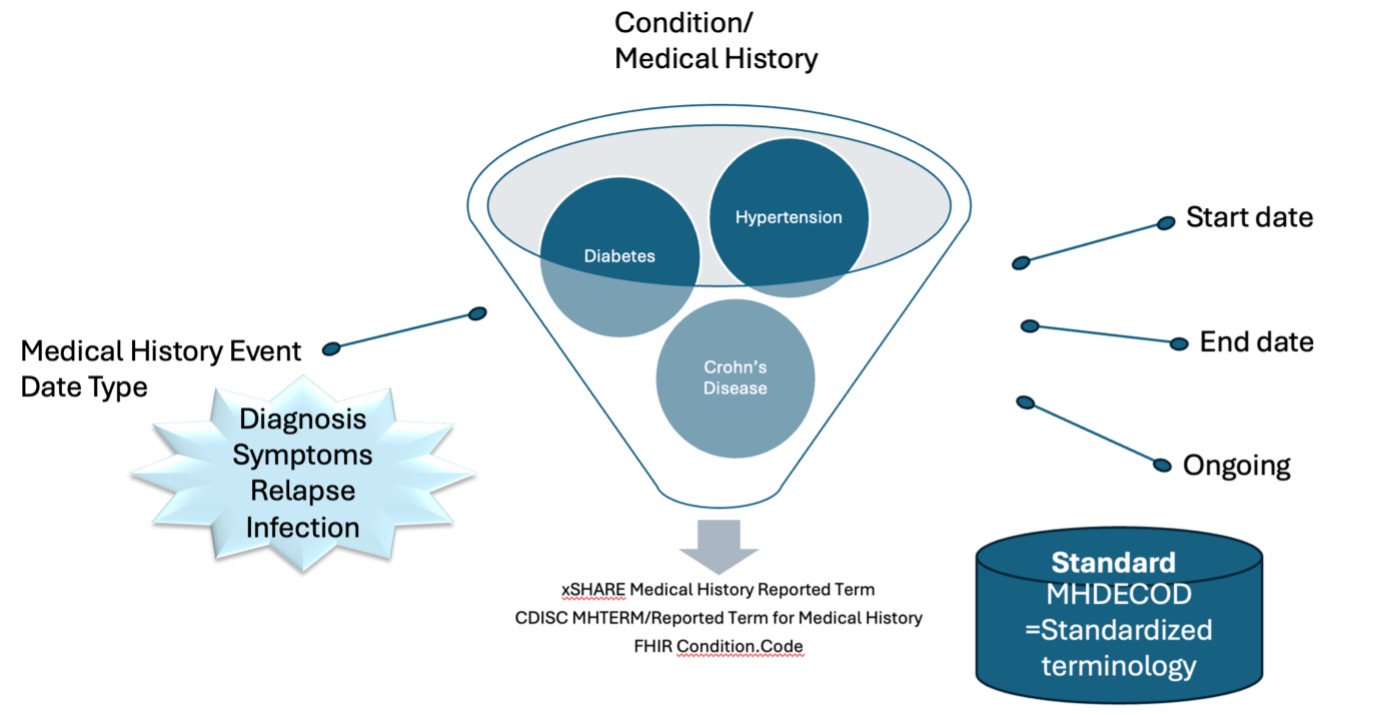
The data domain describes the data model and the data exchange format.
The figure below provides a view of the data elements as recommended in the Proposal for a harmonized core data set across health care, population health and clinical research for the content of the IPS+. These recommendations are the results of:
The data element concepts are aligned with the CDISC Domain and the equivalent IPS Category.

Adverse Events (AEs) in prospective studies are new conditions after trial enrolment, and would likely be collected in EMR/EHR/PHR systems as Conditions. Their classification as AEs is due to the timing relative to study enrolment.
These concepts are central to IPS+ in the context of interventional clinical trials (e.g., treatment efficacy and safety). The collection of AEs and Serious Adverse Events (SAEs) ensures participant safety.
An SAE may include any of the following:
In the context of a drug/device trial, a formal SAE report must assess the impact of the study on the event and guide protective actions.
The SAE report includes:
All these components are included in the IPS+ data model. Automating SAE population in an eCRF via European EHRxF would greatly improve safety workflows.
If new occurrences of conditions or allergies arise in the patient, even during a clinical trial, these data would also be entered in an EHR as new conditions and allergies using its usual data elements. Therefore, in terms of mapping, these additional data element concepts specified for Adverse Events will likely be documented only on the research side and the full Adverse Event data element set does not need to be added to the IPS. Thus, the only additional concepts not in the current IPS are Research Subject, Research Study and the Healthcare Encounter data element set.
To simplify data mapping in PHRs, several CDISC resources can be used:
These tools support transformation from FHIR IPS to the CDISC SDTM specification.
FHIR-CDISC mapping documents and aligned value sets aid in enabling secondary data use from PHRs.
Note that CDISC uses MedDRA codes, whereas the IPS is assumed to use SNOMED CT. A SNOMED CT to MedDRA mapping is maintained and published by SNOMED International. Leveraging the FHIR to CDISC maps, the value sets provided that are aligned with the CDISC terminology will assist in providing support for PHRs that can be leveraged for secondary data use.