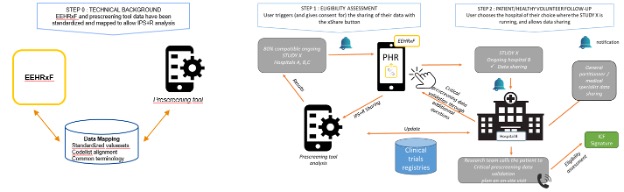
|
Type
|
Reference
|
Content
|
|
web
|
xshare-project.eu
|

|
|
web
|
github.com
|
xShare Project IPS+, published by xShare Project. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/hl7-eu/xshare-ips-plus/
and changes regularly. See the Directory of published versions
|
|
web
|
xshare-project.eu
|
IG © 2024+ xShare Project
. Package hl7.eu.fhir.xshare-ips-plus#0.1.0 based on FHIR 4.0.1
. Generated 2026-02-25
|
|
web
|
facilitate-project.eu
|
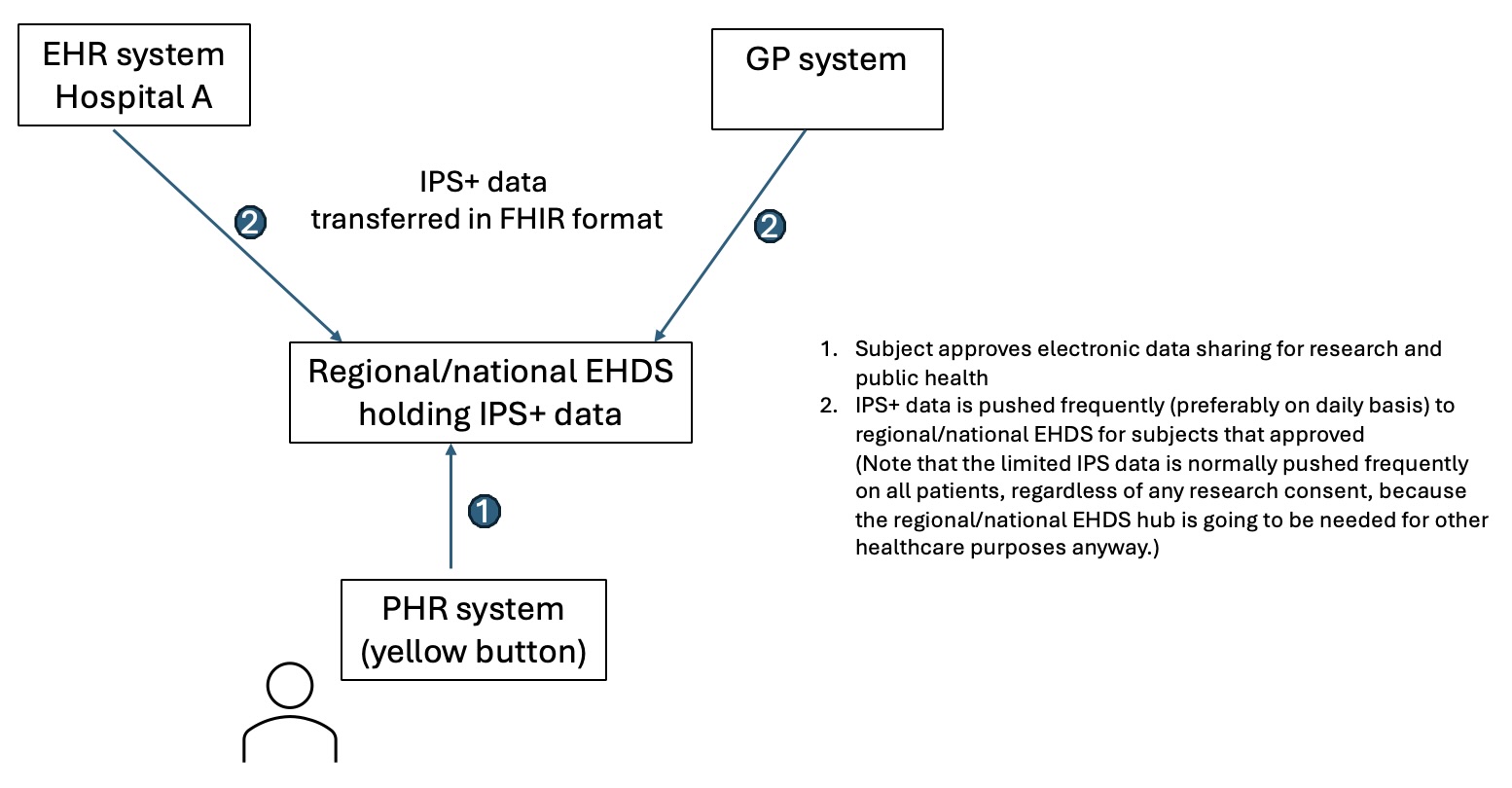
It is recognised that patients can benefit from being able to access and possibly download an identifiable data copy of the data captured during their trial participation, which goes beyond the scope of the IPS+ by covering a much wider range of data elements, sometimes quite specialised disease specific ones. This functionality is beyond the scope of this project, but is being tackled by other European projects, in particular IHI Facilitate
.
|
|
web
|
www.cdisc.org
|
CDISC eCRF Portal
with CDASH-compliant annotated eCRFs
|
|
web
|
www.cdisc.org
|
CDASHIG
providing mappings from CDASH → SDTM
|
|
web
|
profiles.ihe.net
|
Mobile access to Health Documents (MHD), v4.2.2 - Trial-Implementation
|
|
web
|
www.cdisc.org
|
CDISC/TransCelerate Digital Data Flow and The Unified Study Definitions Model
|
|
web
|
www.cdisc.org
|
CDISC Study Data Tabulation Model v2.0 & Study Data Tabulation Model Implementation Guide (SDTMIG) v3.4
|
|
web
|
www.cdisc.org
|
CDISC Clinical Data Acquisition Standards Harmonization Implementation Guides (CDASHIGs) v2.3 (and CDASH Model v1.3)
Includes Case Report Form examples
|
|
web
|
www.cdisc.org
|
CDISC CDASH Serious Adverse Event (SAE) Supplement v2.0
|
|
web
|
www.cdisc.org
|
CDISC Controlled Terminology
(also available in the CDISC Library through the API)
|
|
web
|
www.cdisc.org
|
CDISC Therapeutic Area by Disease
(includes example CRFs and data tables—CDASH, SDTM, ADaM)
|
|
web
|
www.cdisc.org
|
CDISC SDTM and SDTMIG Conformance Rules v2.0
|
|
web
|
www.pinnacle21.com
|
Pinnacle21
(Conformance Rule tool)
|
|
web
|
www.cdisc.org
|
CDISC eCRF Portal
Examples Collection and Articles, includes RedCap link with CDASH forms
|
|
web
|
www.cdisc.org
|
CDISC Library
uses linked data and a REST API to deliver CDISC standards metadata to software applications that automate standards-based processes
|
|
web
|
www.cdisc.org
|
CDISC Data Exchange Standards
|
|
web
|
www.ihe.net
|
IHE International Quality, Research and Public Health
includes integration profiles:
- Retrieve Form for data capture (RFD)
- Retrieve protocol for execution (RPE)
|
|
web
|
www.iso.org
|
Biomedical Research Integrated Domain Group (BRIDG)
and ISO standard
|
|
web
|
github.com
|
OHDSI Clinical Trials Working Group
(draft maps from SDTM to OMOP)
|
|
web
|
health.ec.europa.eu
|
EU Public Health Electronic cross-border health services
|
|
web
|
hl7.eu
|
EU FHIR Extensions
|
|
web
|
hl7.eu
|
EU Laboratory FHIR IG
|