HL7 Europe Common Cancer Model, published by HL7 Europe. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/hl7-eu/cancer-common/ and changes regularly. See the Directory of published versions
A patient’s cancer journey is a longitudinal sequence of events that starts with presentation and evidence gathering, continues through condition assertion and staging, proceeds to treatment (often in overlapping episodes), and is followed by ongoing assessment of response and disease status. Capturing dates consistently for each step is essential so the timeline can be accurately reconstructed for care and research.
Text generated from the picture: to be checked
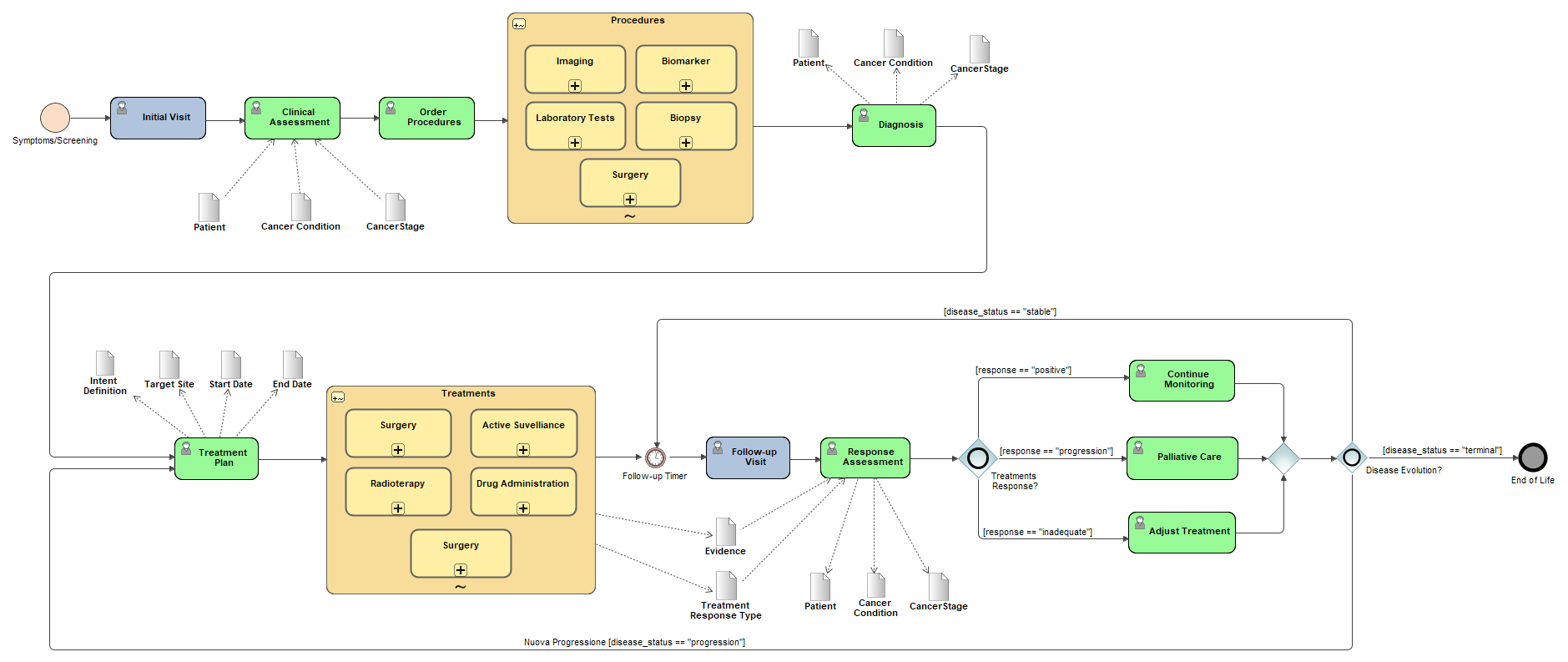
After the initial visit, the patient undergoes a Clinical Assessment.
Key data elements recorded include:
The clinician then proceeds to Order Procedures for further investigation.
Procedures ordered may include:
At this point, the following data are typically updated:
A formal Treatment Plan is created, including:
Treatments may consist of:
During these visits, data are collected:
A Response Assessment is performed to evaluate:
Depending on the outcome of the response assessment:
If the response is positive and disease is stable:
If there is progression of the disease:
Options include:
If the disease status is terminal: