Opioid Prescribing Support Implementation Guide
2016.4.0 - CI Build
Opioid Prescribing Support Implementation Guide
2016.4.0 - CI Build
Opioid Prescribing Support Implementation Guide, published by Centers for Disease Control and Prevention (CDC). This guide is not an authorized publication; it is the continuous build for version 2016.4.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/opioid-cds-r4/ and changes regularly. See the Directory of published versions
| Official URL: http://fhir.org/guides/cdc/opioid-cds/ImplementationGuide/fhir.cdc.opioid-cds-r4 | Version: 2016.4.0 | |||
| Active as of 2022-10-06 | Computable Name: Opioid_CDC | |||
This implementation guide provides resources and discussion in support of applying the 2016 Centers for Disease Control and Prevention (CDC) Guideline:
2016 CDC Guideline for Prescribing Opioids for Chronic Pain
This implementation guide is NOT related to the 2022 Clinical Practice Guideline for Prescribing Opioids for Pain (2022 Clinical Practice Guideline). The 2022 Clinical Practice Guideline updates and replaces the the 2016 Guideline for Prescribing Opioids for Chronic Pain.
This implementation guide was developed based on work initially done as part of the Clinical Quality Framework (CQF) Initiative, a public-private partnership sponsored by the Centers for Medicare & Medicaid Services (CMS) and the U.S. Office of the National Coordinator for Health Information Technology (ONC) to identify, develop, and harmonize standards for clinical decision support and electronic clinical quality measurement, as well as a joint effort by the Centers for Disease Control and Prevention (CDC) and the Office of the National Coordinator for Health IT (ONC) focused on improving processes for the development of standardized, shareable, computable decision support artifacts using the CDC 2016 CDC Guideline as a model case.
Feedback and contributions are welcome and can be submitted using the New Issue link in the footer of every page. Discussions on the use of this IG as well as other CQF projects take place regularly on the CPG-on-FHIR calls, a subworkgroup of the HL7 Clinical Decision Support Workgroup.
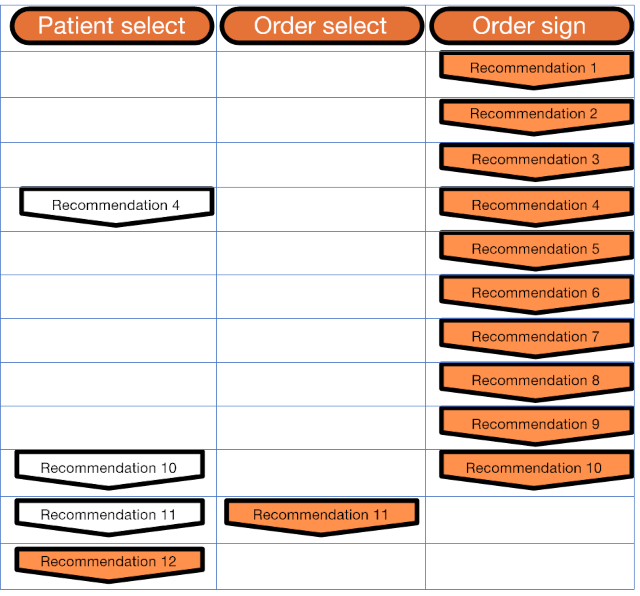
This implementation guide includes support for the following 2016 CDC Guideline recommendations:
For a quick start to get up and running and see how the artifacts work, refer to the Quick Start
For clinical informaticists interested in how the behavior for the artifacts was determined, refer to the Process Documentation.
For an overview of how the artifacts are represented in the FHIR Clinical Reasoning resources, refer to the Integration Documentation.
For a detailed description of how to implement the functionality with CDS Hooks calling into a FHIR Clinical Reasoning server, refer to the Implementation Documentation.
For a detailed description of how to implement the functionality with a Java-based CDS Hooks service, refer to the Service Documentation.
Caution: All doses are in mg/day except for fentanyl, which is mcg/hr.
Caution: Equianalgesic dose conversions are only estimates and cannot account for individual variability in genetics and pharmacokinetics.
Caution: Do not use the calculated dose in MMEs to determine the doses to use when converting one opioid to another; when converting opioids, the new opioid is typically dosed at a substantially lower dose than the calculated MME dose to avoid overdose because of incomplete cross-tolerance and individual variability in opioid pharmacokinetics. Consult the FDA approved product labeling for specific guidance on medications.
Caution: Use particular caution with methadone dose conversions because methadone has a long and variable half-life, and peak respiratory depressant effect occurs later and lasts longer than peak analgesic effect.
Caution: Use particular caution with transdermal fentanyl because it is dosed in mcg/hr instead of mg/day, and its absorption is affected by heat and other factors.
Caution: Buprenorphine products approved for the treatment of pain are not included in the table because of their partial µ-receptor agonist activity and resultant ceiling effects compared with full µ-receptor agonists.
Caution: These conversion factors should not be applied to dosage decisions related to the management of opioid use disorder.
| Opioid | Conversion Factor |
|---|---|
| Codeine | 0.15 |
| Fentanyl transdermal (in mcg/hr) | 2.4 |
| Hydrocodone | 1.0 |
| Hydromorphone | 5.0 |
| Methadone | 4.7 |
| Morphine | 1.0 |
| Oxycodone | 1.5 |
| Oxymorphone | 3.0 |
| Tapentadol 1 | 0.4 |
| Tramadol 2 | 0.2 |
Tapentadol is a µ-receptor agonist and norepinephrine reuptake inhibitor. MMEs are based on degree of µ-receptor agonist activity; however, it is unknown whether tapentadol is associated with overdose in the same dose-dependent manner as observed with medications that are solely µ-receptor agonists. ↩
Tramadol is a µ-receptor agonist and norepinephrine and serotonin reuptake inhibitor. MMEs are based on degree of µ-receptor agonist activity; however, it is unknown whether tramadol is associated with overdose in the same dose-dependent manner as observed with medications that are solely µ-receptor agonists. ↩