Opioid Prescribing Support Implementation Guide, published by Centers for Disease Control and Prevention (CDC). This guide is not an authorized publication; it is the continuous build for version 2016.4.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/opioid-cds-r4/ and changes regularly. See the Directory of published versions
Recommendation #8:
Before starting and periodically during continuation of opioid therapy, clinicians should evaluate
risk factors for opioid-related harms. Clinicians should incorporate into the management plan strategies
to mitigate risk, including considering offering naloxone when factors that increase risk for opioid
overdose, such as history of overdose, history of substance use disorder, higher opioid dosages
(≥50 MME/day), or concurrent benzodiazepine use, are present (recommendation category: A, evidence type: 4).
Functional Description
- When
- Provider is prescribing an opioid analgesic with ambulatory misuse potential in the outpatient setting:
-
Prescription is for treating chronic pain.
- Opioid review is useful for this patient:
- Patient is 18 or over
- Patient does not have findings indicating limited life expectancy
- Patient does not have orders for therapies indicating end of life care
- Patient is not undergoing active cancer treatment:
- Patient has had at least 2 office visits within the past 12 months with an oncology specialist present, or
- Patient has had at least 2 office visits within the past 12 months with a CDC malignant cancer condition diagnosis
- Patient is not currently prescribed naloxone medications
- Factors that increase risk for opioid overdose are present:
- Concurrent benzodiazepine use
- High opioid dosages (MME/day >= 50)
- History of substance abuse
- Then
- Recommend performing opioid misuse assessment:
- Will offer naloxone medications
- Benefits outweigh risks, snooze 3 months
- N/A - see comment, snooze 3 months
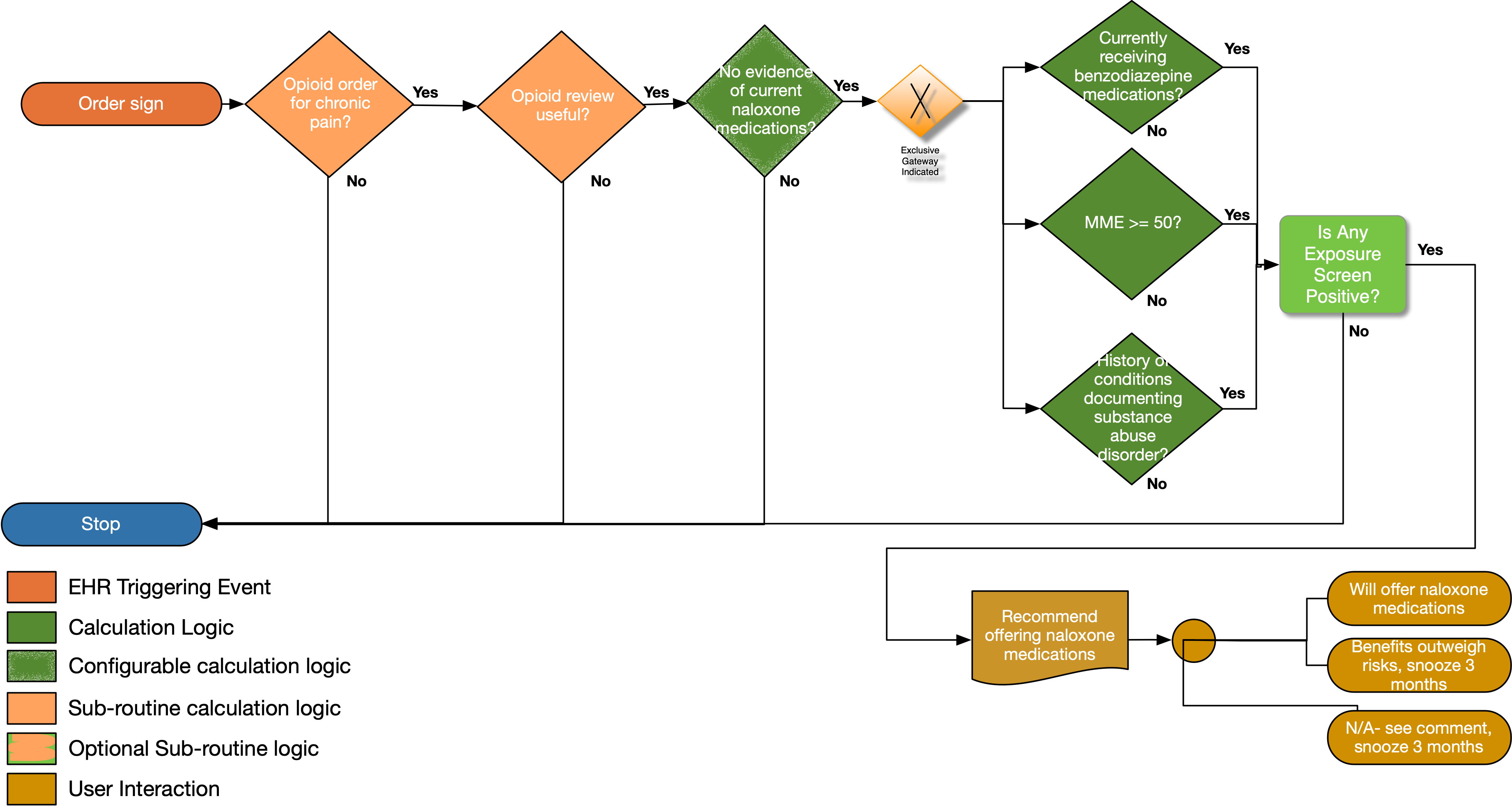
The following table describes the flowchart decisions and sub-routines for the recommendation
| Definition |
Answer to Proceed |
Details |
Data (Terminology) Requirement |
| Opioid order for chronic pain? |
Yes |
See For Chronic Pain sub-routine |
|
| Opioid review useful? |
Yes |
See Opioid Review Useful sub-routine |
|
| No evidence of current naloxone medications? |
Yes |
Find evidence of naloxone medication prescription (An active medication order for naloxone) |
Naloxone medications |
| Currently receiving benzodiazepine medications? |
Yes |
Find evidence of benzodiazepine prescription (An active medication order for benzodiazepine) |
Benzodiazepine medications |
| MME (morphine milligram equivalents > 50) |
Yes |
Determine MME from existing active prescriptions (recommendation 5), or future scope; dispensed medications, or patient-recorded medications |
|
| History of conditions documenting substance misuse disorder? |
Yes |
Find evidence conditions documenting substance misuse in problem list or past medical history |
Conditions documenting substance misuse disorder |
Effective Data Requirements
For Chronic Pain
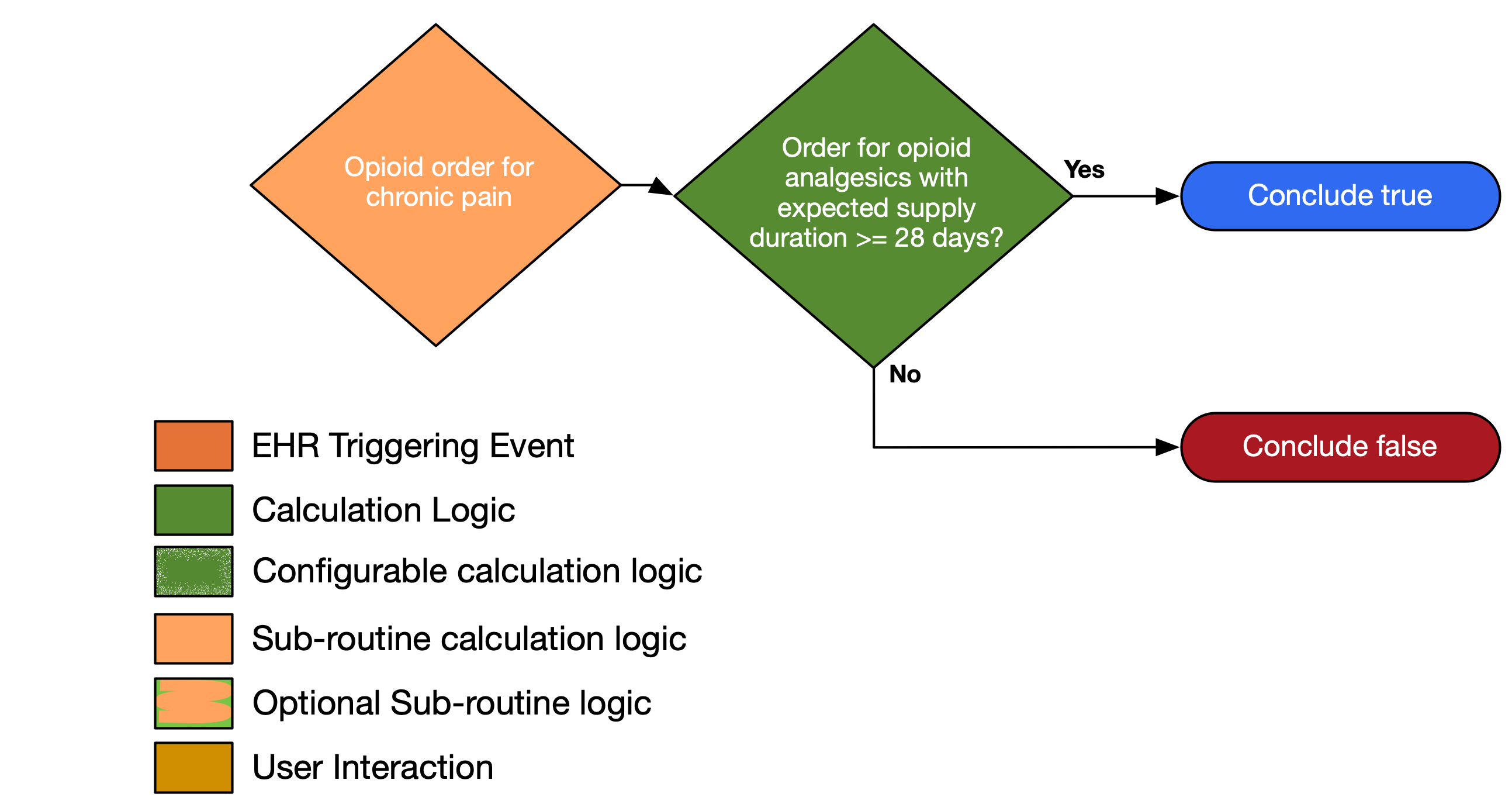
| Definition |
Answer to Proceed |
Details |
Data (Terminology) Requirement |
| Order for opioid analgesic with expected supply duration ≥ 28 days |
Yes |
Order for opioid analgesics with ambulatory misuse potential with a supply duration of ≥ 28 days |
Opioid analgesics with ambulatory misuse potential |
Opioid Review Useful
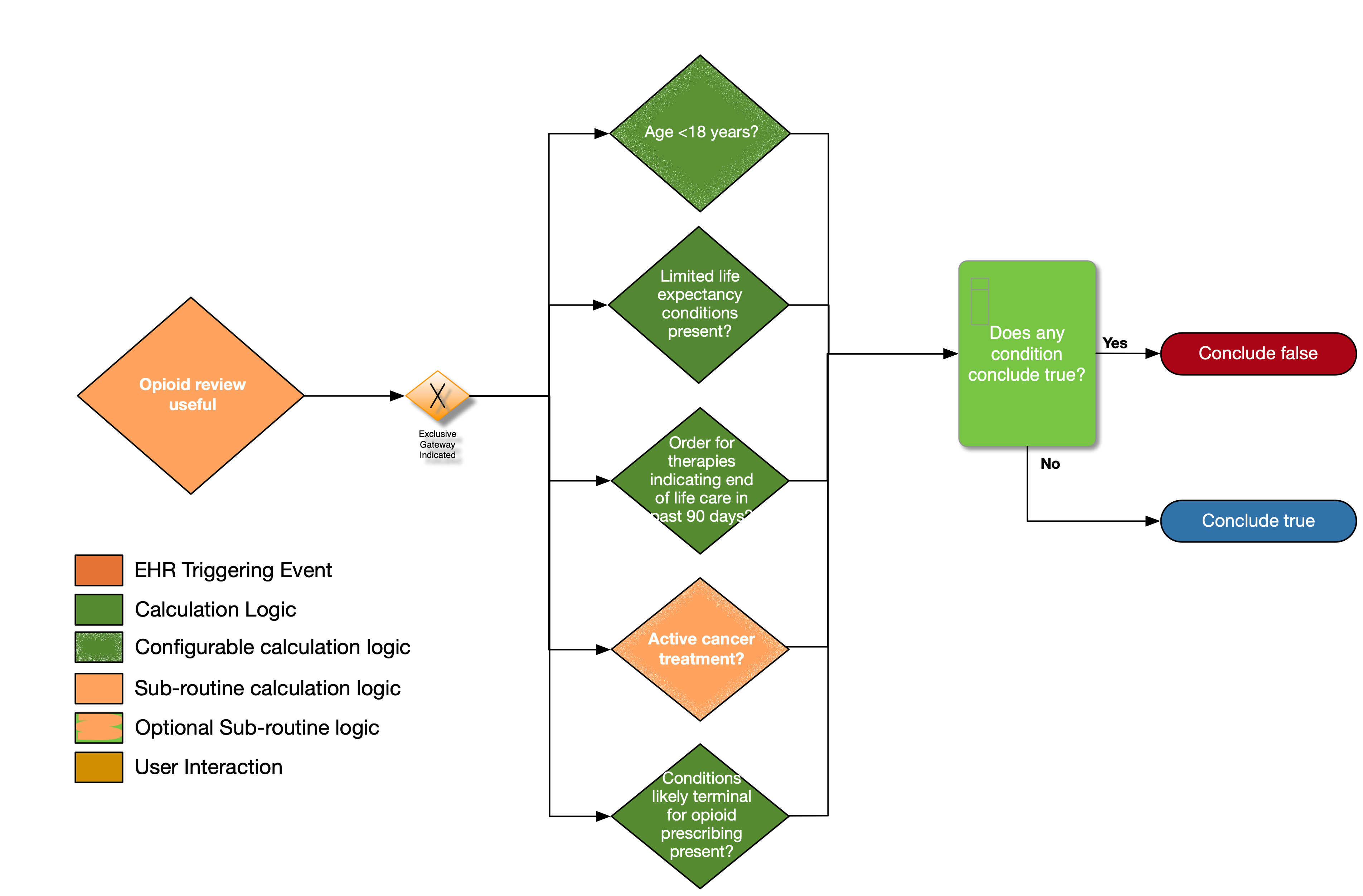
| Definition |
Answer to Proceed |
Details |
Data (Terminology) Requirement |
| Age < 18 years? |
No |
Calculate age from date of birth; exclude patients with age less than 18 years at the time of the prescription |
Date of birth |
| Limited life expectancy conditions present? |
No |
Look for documented findings consistent with those listed in the limited life expectancy value set (terminal illness, bad prognosis, pre-terminal) |
Limited life expectancy conditions |
| Order for therapies indicating end of life care in past 90 days? |
No |
Look for patients with an existing order for therapies indicating end of life care written within past 90 days |
Therapies indicating end of life care |
| Active cancer treatment? |
No |
See Active Cancer Treatment sub-routine |
See Active Cancer Treatment sub-routine |
| Conditions Likely Terminal for opioid prescribing present? |
No |
Look for patients with active conditions in the value set end-of-life-conditions |
Conditions likely terminal for opioid prescribing |
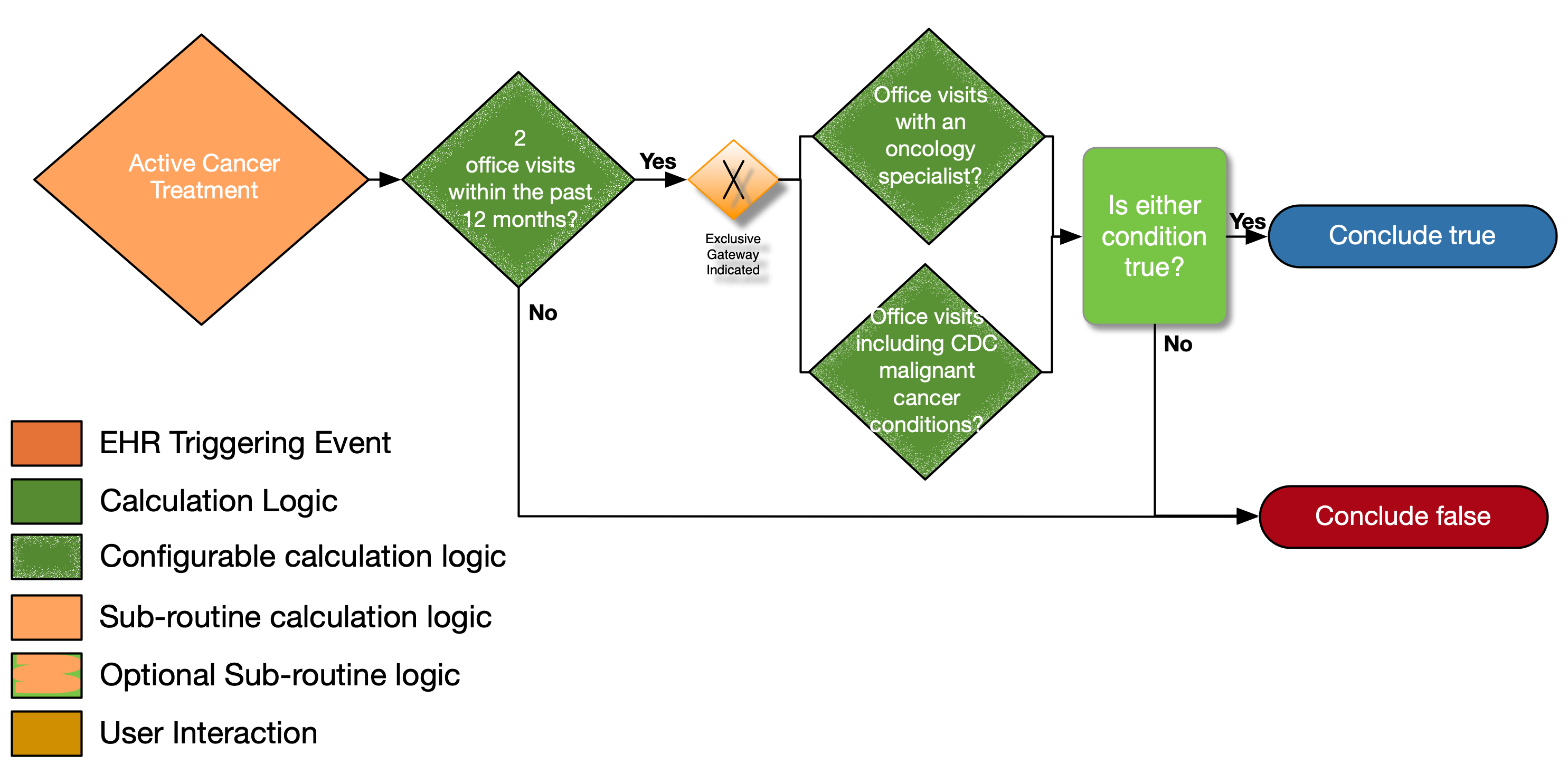
| Definition |
Answer to Proceed |
Details |
Data (Terminology) Requirement |
| Two office visits within the past 12 months? |
No |
Look for a minimum of two distinct encounters within 12 months of the date of the current visit for which each of the following is true:
• the encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set |
Office Visit |
| Office visits with an oncology specialist present? |
No |
• The encounter is performed by an oncologist as defined in the oncology specialty designations using the National Uniform Claim
Committee (NUCC) classifications |
Oncology specialty designations (NUCC) |
| Office visits including CDC malignant cancer condition? |
No |
• The encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set |
CDC malignant cancer conditions |
Content
The following artifacts formalize the description of the logic and behavior defined by this recommendation.
Example Data/Requests
| Description | CDS Hooks Request | Expected Response |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 10 days for 30 days. This will trigger the message "Incorporate into the management plan strategies to mitigate risk; including considering offering naloxone when factors that increase risk for opioid overdose are present." Consider offering naloxone given following risk factor(s) for opioid overdose: Average MME (54.000000 'mg/d') >= 50 mg/day. |
Request JSON |
Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. |
Request JSON |
Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. Patient has also been prescribed Temazepam 20 MG Oral Tablet (RXNorm 104693) 1 tablet per 1 day for 30 days. This will trigger the message “Incorporate into the management plan strategies to mitigate risk; including considering offering naloxone when factors that increase risk for opioid overdose are present. Consider offering naloxone given following risk factor(s) for opioid overdose: concurrent use of benzodiazepine. |
Request JSON |
Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. Patient has also been prescribed Naloxone Hydrochloride 0.02 MG/ML Injectable Solution (RXNorm 1191212) 1ml per 1 day for 30 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. |
Request JSON |
Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. This will trigger the message "Incorporate into the management plan strategies to mitigate risk; including considering offering naloxone when factors that increase risk for opioid overdose are present" Consider offering naloxone given following risk factor(s) for opioid overdose: history of alcohol or drug abuse. |
Request JSON |
Response JSON |