UMC IDMP Request and Publish API, published by Uppsala Monitoring Centre. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/Uppsala-Monitoring-Centre/WHO-UMC-IDMP-Service/ and changes regularly. See the Directory of published versions
IDMP – Identification of Medicinal Products – is a set of five standards developed by the International Organization for Standardization (ISO) to create a universal framework of structured, coded data that uniquely identify and describe all key aspects of medicinal products. The Identification of Medicinal Products (IDMP) standards aim to increase clarity and efficiency in communications about medicines and provide greater certainty to patients no matter where they are.
The UMC IDMP API supports two parts of the ISO IDMP standard, the Pharmaceutical Product Identifier (PhPID) and the Global Substance Identifier (GSID):
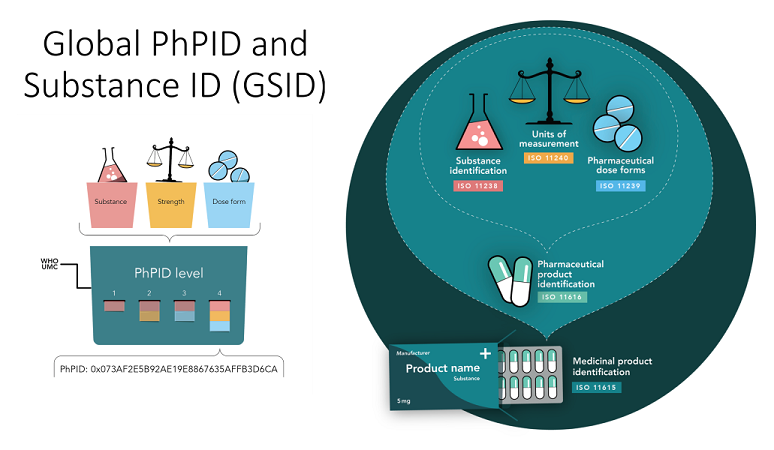
The PhPID is based on substance-, strength- and dose form information of the pharmaceutical product. This information, once validated and harmonized through a set of business rules developed by the GIDWG, is used as input to the generation of the PhPID. PhPIDs of four different levels are generated, as follows: