Order Catalog Implementation Guide, published by HL7 International / Orders and Observations. This guide is not an authorized publication; it is the continuous build for version 1.0.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/fhir-order-catalog/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
This page provides the detailed specifications for catalogs of medical devices
The figure below shows the resources and profiles used to represent catalogs of medical devices.
Figure 9.1: Artifacts used in catalogs of devices
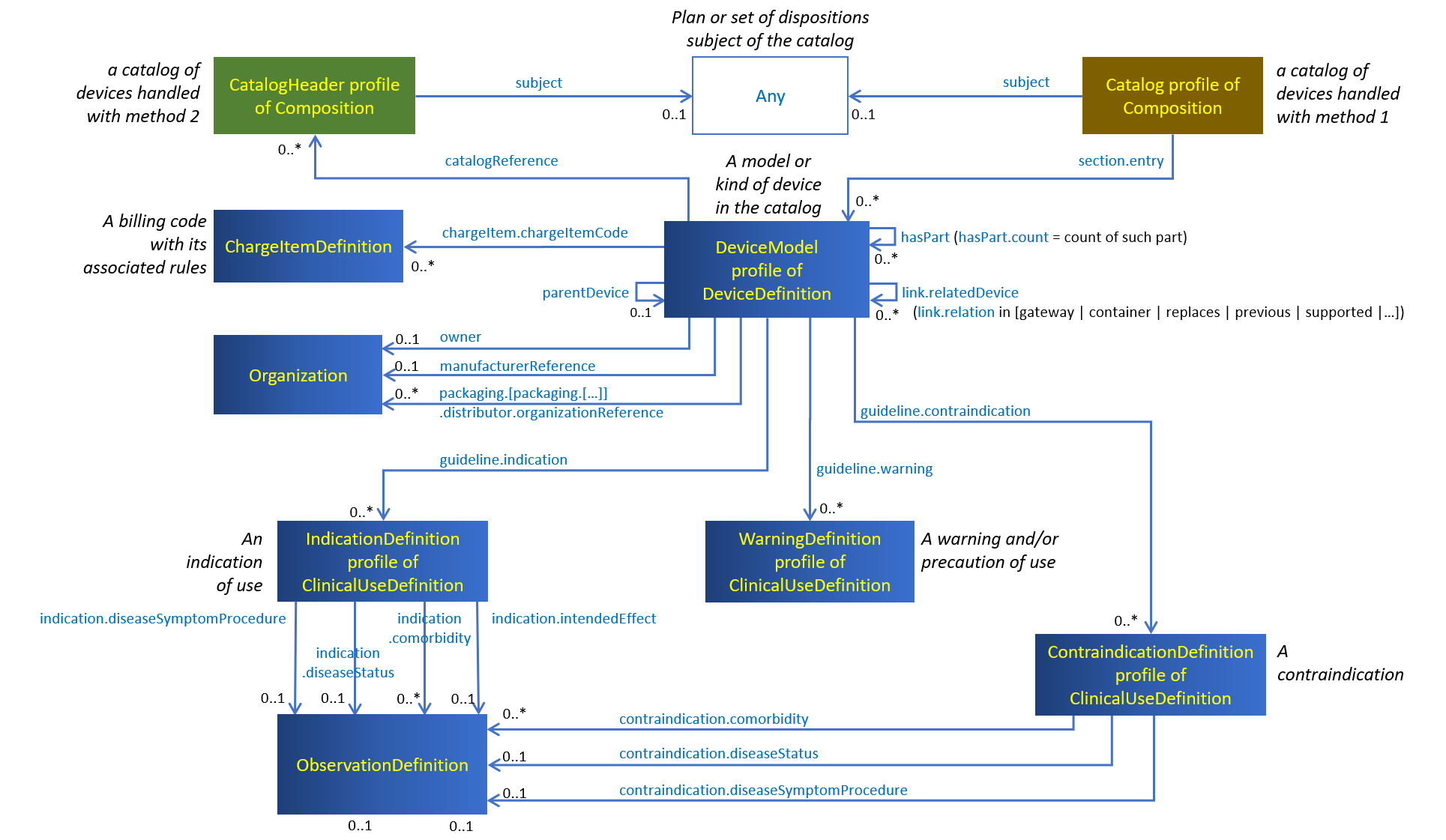
When method 1 is chosen by the custodian of the catalog of devices, the catalog references its items: the Composition resource constrained by the Catalog profile to represent the whole catalog, references the items of this catalog from its Composition.section.entry elements.
When method 2 is chosen instead, the catalog is referenced by its items: Each DeviceDefinition resource constrained by the DeviceModel profile and representing an item of the catalog, references the Composition resource constrained by the CatalogHeader profile to represent the catalog header, which holds the catalog general properties.
An item of the catalog describes a model or type of device, with its various identifiers, classifications, safety characteristics and properties, instantiated as a DeviceDefinition resource linked to a number of supporting resources providing further details about this item.
The key searcheable assets in a catalog of devices are the model of devices exposed to the consumers as instances of DeviceDefinition.
_include:iterate=* parameter, to retrieve the selected DeviceDefinition resource(s) with their supporting resources (ClinicalUseDefinition, ChargeItemDefinition) in the searchset Bundle._include:iterate=* parameter so as to obtain all the supporting resources of each device retrieved, in the searchset Bundle.Catalog servers may limit the iteration depth to an appropriate level for performance sake.
| Name | Type | Description | Expression | Comment |
|---|---|---|---|---|
| _lastUpdated | date | Last system point in time of the DeviceDefinition instance | can be used with =gt… | |
| class | token | specific class of models of devices | DeviceDefinition.classification.type | For instance device class in GMDN or EMDN |
| udi | token | The primary UDI of the device model | DeviceDefinition.udiDeviceIdentifier.deviceIdentifier | |
| packaging-udi | token | The primary UDI of the package containing the device | DeviceDefinition.packaging.udiDeviceIdentifier.deviceIdentifier | |
| identifier | token | A business identifier (other than primary UDI) of the device | DeviceDefinition.identifier | |
| model-number | string | The model number of the device | DeviceDefinition.modelNumber | |
| name | string | a name of the model of device | DeviceDefinition.deviceName.name | A device may have more than one name. |
| catalog | reference | The reference to a Composition resource (profiled by CatalogHeader) owning this item | DeviceDefinition.extension.where(url='http://hl7.org/fhir/uv/order-catalog/StructureDefinition/CatalogReference').valueReference.reference(Composition) | catalog to search from |
In all examples below, base represents the endpoint of the catalog server. The answer of the server comes as a Bundle of type 'searchset' encapsulating the resources selected by the search. One particular device catalog is assumed to have Composition.id "a1" on the server.
GET base/Composition?type:text=Catalog&_summary=true
Obtains the summary of every catalog available on the server. The anwser Bundle contains one entry with a Composition resource for each catalog found.
GET base/Composition?type:text=Catalog&category=device&_summary=true
Obtains the summary of each catalog of devices available on the server. The anwser Bundle contains one entry with a Composition resource for each device catalog found.
GET [base]/DeviceDefinition?modelNumber=SCF332&_include:iterate=*
Obtains the full content of instances of DeviceDefinition with modelNumber = "SCF332" accompanied by their supporting resources. The anwser Bundle contains the full details for each: An entry for each matching DeviceDefinition and, below it, as many entries as resources referenced by this one (recursively).
| GUDID | EUDAMED | DeviceDefinition | Device |
|---|---|---|---|
| Brand Name | FLD-UDID-22: Device Name (under Basic UDI) | deviceName (type = registered-name) | deviceName (type = registered-name) |
| n.a. | FLD-UDID-176: Name/Trade name(s) | deviceName (type = user-friendly-name) | deviceName (type = user-friendly-name) |
| Version or Model | FLD-UDID-20: Device model (under Basic UDI) | modelNumber | modelNumber |
| Catalog Number | FLD-UDID-163: Reference-catalogue number | partNumber | partNumber |
| Primary DI Number | FLD-UDID-178: UDI-DI code | udiDeviceIdentifier.deviceIdentifier | udiCarrier.deviceIdentifier |
| Issuing Agency (for Primary DI Number) | FLD-UDID-291: Issuing Entity UDI-DI | udiDeviceIdentifier.issuer | udiCarrier.issuer |
| Device description | FLD-UDID-175: Additional Product description | description | n.a. |
| Company Name | FLD-UDID-353: Actor/Organisation name | manufacturer.display or manufacturer.reference->Organization.name | manufacturer |
| Secondary DI Number | FLD-UDID-136: Secondary UDI-DI code | identifier.value (use = secondary, type = UDI) | identifier.value (use = secondary, type = UDI) |
| Issuing Agency (for Secondary DI Number) | FLD-UDID-293: Issuing Entity Secondary DI | identifier.assigner (use = secondary, type = UDI) | identifier.assigner (use = secondary, type = UDI) |
| DM DI Number | FLD-UDID-138: Direct Marking UDI-DI code | identifier.value (use = official, type = DIRECT-MARKING) | identifier.value (use = official, type = DIRECT-MARKING) |
| n.a. | FLD-UDID-294: Issuing Entity Direct marking DI | identifier.assigner (use = official, type = DIRECT-MARKING) | identifier.assigner (use = official, type = DIRECT-MARKING) |
| Unit of Use DI Number | FLD-UDID-135: Unit of Use DI code | identifier (use = official, type = SINGLE-ITEM) | identifier (use = official, type = SINGLE-ITEM) |
| n.a. | FLD-UDID-14: Basic UDI-DI | regulatoryIdentifier (type = basic) | n.a. |
| PACKAGE DI Number | FLD-UDID-120: Package UDI-DI | packaging[.packaging]*.udiDeviceIdentifier.deviceIdentifier | n.a. |
| Commercial Distribution Status | FLD-UDID-130: Device status (on EU market, no longer on EU market, not intended for EU market) | inferred from marketDistribution.marketPeriod | n.a. |
| n.a. | FLD-UDID-250: Start date (per EU member country where device is commercialized) | marketDistribution.marketPeriod.start | n.a. |
| Commercial Distribution End Date | FLD-UDID-251: End date (per EU member country where device is commercialized) | marketDistribution.marketPeriod.end | n.a. |
| n.a. | FLD-UDID-252: Member State were the device is or is to be made available | marketDistribution.subJurisdiction | n.a. |
| Device Count (Number of MDs contained in the base package) | FLD-UDID-121: Quantity of item(s)(for any package level, including the base package) | packaging.count | n.a. |
| Labeler D-U-N-S Number | FLD-UDID-10: Legal manufacturer SRN | manufacturerReference->Organization.identifier (type = PRN, system = appropriate namespace for D-U-N-S in the US or SRN in EU) | n.a. |
| n.a. | FLD-UDID-16: Risk Class | classification.type (system representing Risk Class code system) | n.a. |
| Device GMDN classification | n.a. | classification.type (system = http://terminology.hl7.org/CodeSystem/GMDN) | n.a. |
| n.a. | Device EMDN classification | classification.type (system = urn:oid:1.2.250.1.213.2.68) | n.a. |
| Previous DI (same version or model of device) | ? | ? | n.a. |
| MRI safety information: safe, unsafe, conditional, not stated (ASTM F2503) | FLD-UDID-144: List of critical warnings (FLD-UDID-212: Critical warnings type) : safe, unsafe, conditional, consider hazards for magnetic fields, do not store near magnets or magnetic devices | safety: ValueSet device-safety (ASTM F2503 with C-codes from NCIthesaurus) | safety: ValueSet device-safety (ASTM F2503 with C-codes from NCIthesaurus) |
| Device required to be labeled as containing latex (Y/N) | ? | ? | n.a. |
| Device labeled as containing latex | FLD-UDID-156: Containing latex (Boolean) | safety: ValueSet device-safety (ASTM F2503 with C-codes from NCIthesaurus) | safety: ValueSet device-safety (ASTM F2503 with C-codes from NCIthesaurus) |
| For Single Use | ? | property | n.a. |
| Prescription Use | ? | property | n.a. |
| Over the Counter | ? | property | n.a. |
| Human Cell, Tissue or Cellular or Tissue-Based Product | ? | property | n.a. |
| n.a. | FLD-UDID-28: Active Device | property | n.a. |
| n.a. | FLD-UDID-30: Implantable | property | n.a. |
See the LIVD Implementation Guide.