HL7 Czech Imaging Report IG
0.1.0-ballot - ci-build
HL7 Czech Imaging Report IG
0.1.0-ballot - ci-build
HL7 Czech Imaging Report IG, published by HL7 Czech Republic. This guide is not an authorized publication; it is the continuous build for version 0.1.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7-cz/img/ and changes regularly. See the Directory of published versions
A typical, standard course of the examination takes place gradually, where the individual steps follow each other in sequence, as shown in the following figure of the sequence diagram. This diagram does not include the extended Imaging Methods Report and its storage in the EHR repository and sending to the external examination orderer.
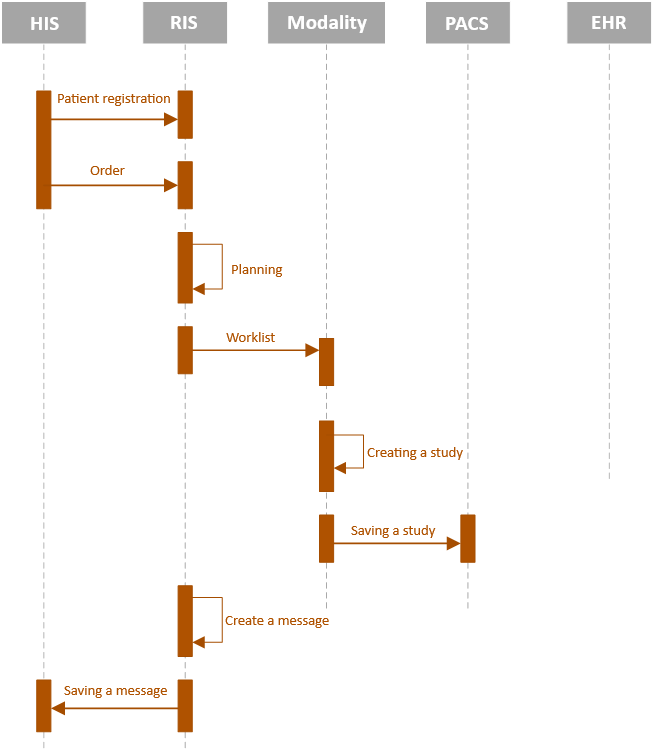
Fig. 1: STANDARD WORKFLOW FOR IMAGING METHODS
The individual phases of the examination represent the state of development of the examination. The phase, the state in which the examination is, is informed by the so-called DICOM Modality Performed Procedure Step (MPPS) messages.
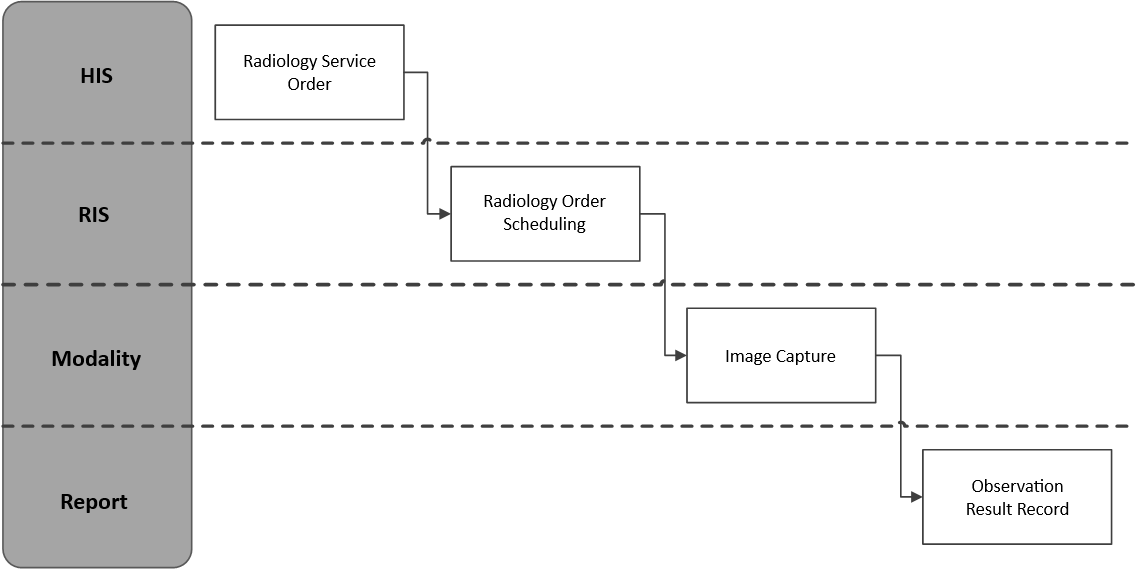
Fig. 2: PHASE AND STATES OF THE EXAMINATION PROCESS
The target concept of the extended Report from imaging methods, including storage in the EHR repository, is shown in the following figure. The actor "healthcare professional" (HP) is either the indicating physician, radiology assistant or physician - radiologist, depending on the interaction with the relevant IS.
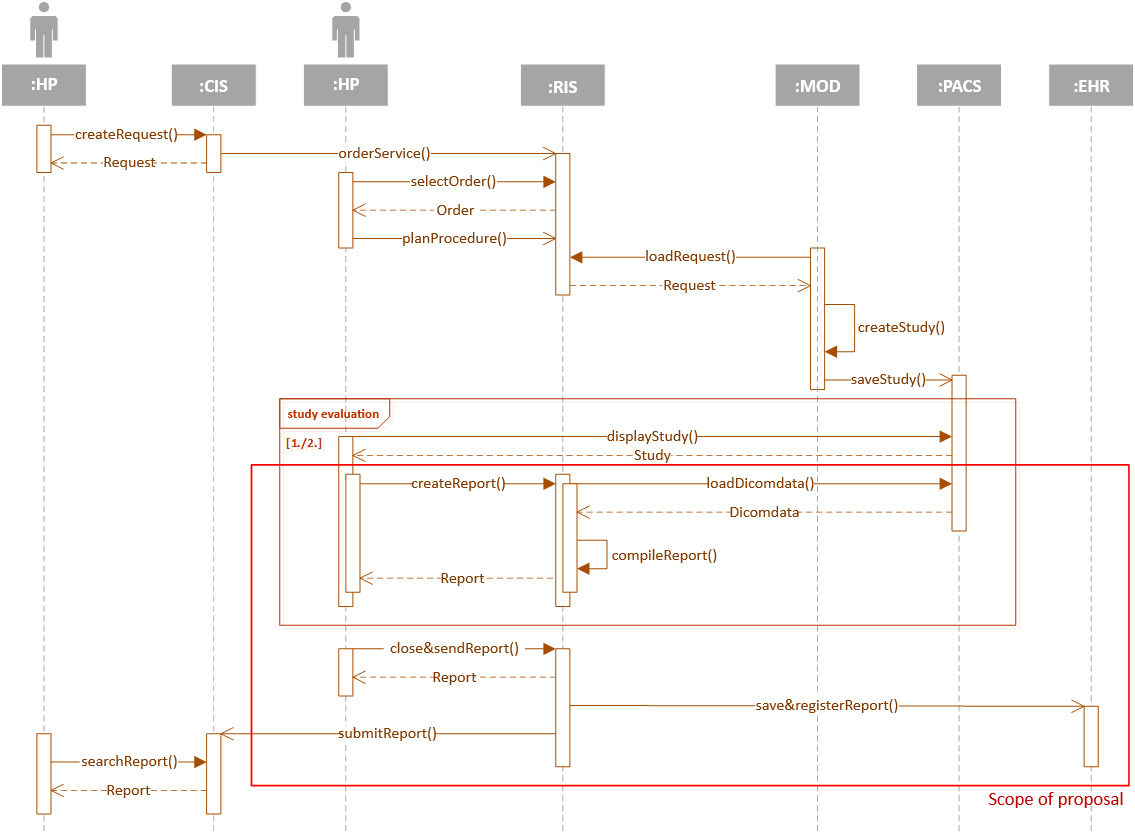
Fig. 3: EXTENDED CONCEPT OF REPORTING FROM IMAGING METHODS