HL7 Czech Hospital Discharge Report Implementation Guide, published by HL7 Czech Republic. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7-cz/hdr/ and changes regularly. See the Directory of published versions
Domovská stránka
Úvod
Příručka stanovuje sadu pravidel, která budou použita pro HL7 FHIR k definování způsobu reprezentace propouštěcí zprávy z nemocnice v českém národním kontextu, v souladu s evropskými směrnicemi eHN.
Hlavním cílem je definovat obsahové složky a preferovanou strukturu pro sestavení propouštěcí zprávy z nemocnice. Účelem tohoto standardu je definovat reprezentaci propouštěcí zprávy jako součásti zdravotnické dokumentace pacienta za účelem elektronické výměny zdravotních informací mezi jednotlivci, poskytovateli zdravotní péče a infrastrukturou v České republice.
Toto zahrnuje jak národní, tak přeshraniční scénáře.
Tato příručka nepopisuje, jak tuto zprávu elektronicky vyměňovat.
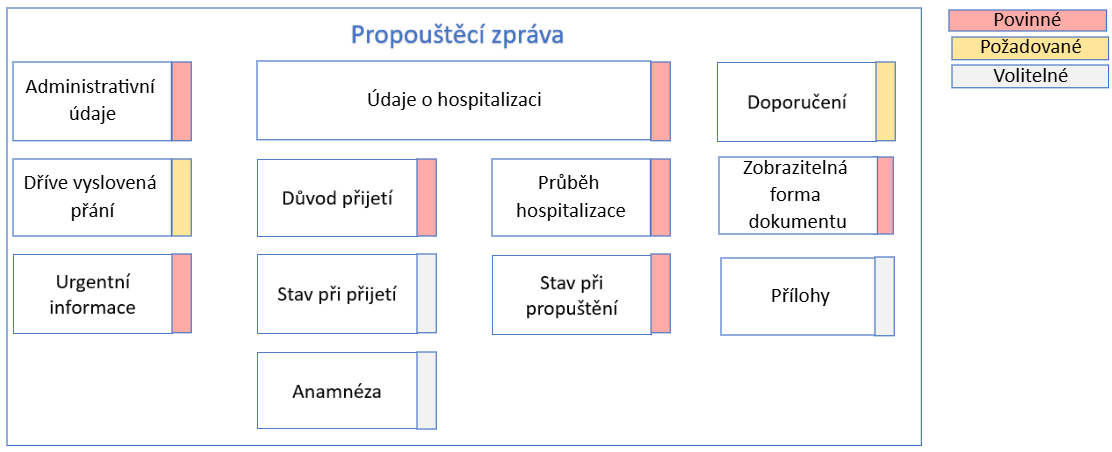
Fig. 1: Struktura propouštěcí zprávy
Základní sekce propouštěcí zprávy z nemocnice
Rozsah
Tento dokument představuje koncepty českého použití definované prostřednictvím FHIR artefaktů, které lze strojově zpracovat. Jedná se o výsledky spolupráce, ve kterých byly dohodnuty přístupy k různým typům zdravotnických informací, vycházející ze základní specifikace FHIR R4.
Tato příručka je rozdělena do několika stránek, které jsou uvedeny v horní části každé stránky v nabídce:
- Home: Tato stránka poskytuje úvod a rozsah této příručky.
- Artifacts: Tato stránka uvádí podrobné popisy a formální definice všech českých HDR artefaktů a logického modelu definovaného v této příručce.
Závislosti
Package hl7.fhir.uv.extensions.r4#5.2.0
This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Mon, Feb 10, 2025 21:45+1100+11:00)
|
Package hl7.fhir.uv.ipa#1.1.0
This IG describes how an application acting on behalf of a patient can access information about the patient from an clinical records system using a FHIR based API. The clinical records system may be supporting a clinical care provider (e.g. a hospital, or a general practitioner), or a health data exchange, including a national health record system. (built Wed, Mar 19, 2025 14:34+0000+00:00)
|
Package hl7.fhir.eu.extensions#0.1.1-ballot
This guide lists the extensions speciifed for the European REALM. (built Wed, Apr 30, 2025 13:56+0200+02:00)
|
Package hl7.fhir.uv.ips#1.1.0
International Patient Summary (IPS) FHIR Implementation Guide (built Tue, Nov 22, 2022 03:24+0000+00:00)
|
Package hl7.fhir.eu.laboratory#0.1.1
This guide describes how the Laboratory Report can be represented in the European REALM. (built Tue, Mar 25, 2025 12:00+0100+01:00)
|
Package hl7.fhir.eu.extensions.r4#0.1.1-ballot
This guide lists the extensions speciifed for the European REALM. (built Wed, Apr 30, 2025 13:56+0200+02:00)
|
Package hl7.fhir.eu.base#0.1.0-ballot
This guide collects base and core profiles to be used in the European context. It also includes common artifacts, such as the profiles describing the European Health Insurance Card. (built Mon, May 5, 2025 21:42+0200+02:00)
|
Package ihe.pharm.mpd.r4#1.0.0-comment-2
ImplementationGuide for IHE Pharmacy Medication Prescription and Dispense (MPD) profile (built Tue, May 27, 2025 16:32+0200+02:00)
|
Package hl7.fhir.eu.hdr#0.1.0-ballot
This implementation guide specifies the Hospital Discharge Report in the European context, as defined in eHN Hospital Discharge Report guidelines. (built Tue, Jun 3, 2025 12:48+0200+02:00)
|
Package ihe.pharm.mpd#1.0.0-comment-2
ImplementationGuide for IHE Pharmacy Medication Prescription and Dispense (MPD) profile (built Tue, May 27, 2025 16:32+0200+02:00)
|
Package hl7.fhir.cz.terminology#current
HL7 Czech Terminology Implementation Guide (built Tue, Dec 23, 2025 17:10+0000+00:00)
|
Package hl7.fhir.cz.core#current
HL7 Czech Base and Core Resources Implementation Guide (built Fri, Dec 19, 2025 09:24+0000+00:00)
|
Package hl7.fhir.eu.extensions#current
This guide lists the extensions specified for the European REALM. (built Fri, Dec 19, 2025 13:26+0000+00:00)
|
Package hl7.fhir.uv.xver-r5.r4#0.0.1-snapshot-2
The cross-version extensions available in FHIR R4 from FHIR R5 (built Sat, Sep 13, 2025 16:55-0400-04:00)
|
Package hl7.fhir.eu.extensions.r4#1.2.0
This guide lists the extensions specified for the European REALM. (built Fri, Dec 19, 2025 11:52+0100+01:00)
|
Package hl7.fhir.eu.base#current
This guide collects base and core profiles to be used in the European context. It also includes common artifacts, such as the profiles describing the European Health Insurance Card. (built Wed, Feb 25, 2026 08:21+0000+00:00)
|
Package hl7.fhir.eu.laboratory#current
This guide describes how the Laboratory Report can be represented in the European REALM. (built Mon, Feb 23, 2026 14:45+0000+00:00)
|
Package hl7.fhir.cz.lab#current
Czech Laboratory Implementation Guide (built Wed, Dec 10, 2025 22:15+0000+00:00)
|
Package hl7.fhir.cz.img-order#current
HL7 Czech Imaging Order Implementation Guide (built Wed, Jan 28, 2026 16:09+0000+00:00)
|
Package ihe.iti.balp#1.1.4
The Basic Audit Log Patterns (BALP) Implementation Guide is a Content Profile that defines some basic and reusable AuditEvent patterns. This includes basic audit log profiles for FHIR RESTful operations to be used when there is not a more specific audit event defined. A focus is enabling Privacy centric AuditEvent logs that hold well formed indication of the Patient when they are the subject of the activity being recorded in the log. Where a more specific audit event can be defined it should be derived off of these basic patterns. (built Fri, Oct 31, 2025 12:53-0500-05:00)
|
Package ihe.iti.mhd#4.2.3
ImplementationGuide for IHE IT Infrastructure Technical Framework Supplement Mobile access to Health Documents (MHD) (built Fri, Oct 31, 2025 14:32-0500-05:00)
|
Package hl7.fhir.eu.imaging-r4#current
This guide describes how a Imaging Report can be represented in the European REALM using FHIR R4. (built Thu, Feb 26, 2026 15:28+0000+00:00)
|
Package hl7.fhir.cz.img#current
Czech Imaging Report Implementation Guide (built Wed, Jan 28, 2026 16:02+0000+00:00)
|
Package hl7.fhir.uv.tools.r4#0.9.0
This IG defines the extensions that the tools use internally. Some of these extensions are content that are being evaluated for elevation into the main spec, and others are tooling concerns (built Tue, Dec 16, 2025 23:18+1100+11:00)
|
Analýza mezi verzemi
This is an R4 IG. None of the features it uses are changed in R4B, so it can be used as is with R4B systems. Packages for both R4 (hl7.fhir.cz.hdr.r4) and R4B (hl7.fhir.cz.hdr.r4b) are available.
Prohlášení k právům duševního vlastnictví
This publication includes IP covered under the following statements.
- ISO maintains the copyright on the country codes, and controls its use carefully. For further details see the ISO 3166 web page: https://www.iso.org/iso-3166-country-codes.html
Show Usage
- ISO 3166-1 Codes for the representation of names of countries and their subdivisions — Part 1: Country code: AdvanceDirectives2FHIRCzHdr, Alerts2FHIRCzHdr... Show 127 more, AttachmentHDR2FHIRcz, BirthSummary2FHIRCzHdr, Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1, Bundle/DischargeBundle-Novak-Petr-L1-core, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CHIR - Oddělení chirurgie, CHIR-L2 - Lůžková stanice 2, CZConsentCategory, CZProvisionCode, CZ_AddressObligationsHdr, CZ_AllergyIntoleranceHdr, CZ_AllergyIntoleranceObligationsHdr, CZ_AttachmentHdr, CZ_AttachmentsObligationsHdr, CZ_BundleHdr, CZ_CarePlanHdr, CZ_CompositionHdr, CZ_CompositionObligationsHdr, CZ_ConditionHdr, CZ_ConsentHdr, CZ_ConsentObligationsHdr, CZ_CoverageObligationsHdr, CZ_DeviceUseStatementHdr, CZ_DosageHospitalStayObligationsHdr, CZ_DosageMedicationSummaryObligationsHdr, CZ_EncounterHdr, CZ_EncounterObligationsHdr, CZ_FamilyMemberHistoryHdr, CZ_FlagHdr, CZ_FlagObligationsHdr, CZ_ImmunizationHdr, CZ_ImmunizationObligationsHdr, CZ_ImmunizationRecommendationHdr, CZ_ImmunizationRecommendationObligationsHdr, CZ_MedicalDeviceObligationsHdr, CZ_MedicationAdministrationObligationsHdr, CZ_MedicationDispenseHdr, CZ_MedicationDispenseHospitalStayObligationsHdr, CZ_MedicationDispenseMedicationSummaryObligationsHdr, CZ_MedicationHospitalStayObligationsHdr, CZ_MedicationMedicationSummaryObligationsHdr, CZ_MedicationRequestHdr, CZ_MedicationRequestHospitalStayObligationsHdr, CZ_MedicationRequestMedicationSummaryObligationsHdr, CZ_MedicationStatementObligationsHdr, CZ_ObservationAbdominalCircumferenceHdr, CZ_ObservationAnthropometricDataHdrObligations, CZ_ObservationBMIHdr, CZ_ObservationBirthLength, CZ_ObservationBirthWeight, CZ_ObservationChestCircumferenceHdr, CZ_ObservationFetalPresentation, CZ_ObservationGravidity, CZ_ObservationHeadCircumferenceHdr, CZ_ObservationHeightHdr, CZ_ObservationInfectiousContactHdr, CZ_ObservationInfectiousContactHdrObligations, CZ_ObservationMultiplePregnancy, CZ_ObservationParity, CZ_ObservationSdohHdr, CZ_ObservationTravelHdr, CZ_ObservationWeightHdr, CZ_OrganizationObligationsHdr, CZ_PatientObligationsHdr, CZ_PractitionerObligationsHdr, CZ_PractitionerRoleObligationsHdr, CZ_ProcedureHdr, CZ_ProcedureInductionOfLabor, CZ_ProcedureMethodOfDelivery, CZ_ProcedureObligationsHdr, CZ_RelatedPersonHdr, CZ_RelatedPersonObligationsHdr, EHDSIConditionPOA, EHDSITreatmentClass, Encounter2FHIRCzHdr, ExposureAgentHdrVs, FunctionalStatus2FHIREuHdr, HL7CzHospitalDischargeIg, Hdr2FHIRCzHdr, Header2FHIRczHdr, HospitalStay2FHIRCzHdr, LMCzAdmissionEvaluation, LMCzAdvanceDirectivesEhnCz, LMCzAlertsCz, LMCzAttachmentsCz, LMCzBirthSummaryHdrCz, LMCzDischargeDetailsCz, LMCzEncounterCz, LMCzFunctionalStatusHdrCz, LMCzHeaderCz, LMCzHospitalDischargeReportCz, LMCzHospitalStayCz, LMCzMedicationSummaryHdrCz, LMCzPatientHistoryCz, LMCzPlanOfCareHdrCz, LMCzPresentedFormCz, LMEnAdmissionEvaluationEhnEn, LMEnAdvanceDirectivesEhnCz, LMEnAlertsEhnCz, LMEnAttachmentsCz, LMEnBirthSummaryHdrCz, LMEnDischargeDetailsEhnCz, LMEnEncounterEhnCz, LMEnFunctionalStatusHdrEhnCz, LMEnHeaderHdrCz, LMEnHospitalDischargeReportEhnCz, LMEnHospitalStayEhnCz, LMEnMedicationSummaryHdrEhnCz, LMEnObjectiveFindingsHdrEhnCz, LMEnPatientHistoryEhnCz, LMEnPlanOfCareHdrEhnCz, LMEnPresentedFormCz, MedicationSummary2FHIRCzHdr, Nemocnice Chrudim, Nemocnice Pardubického kraje, a.s., Chrudimská nemocnice, ObjectiveFindings2FHIREuHdr, ObjectiveFindingsHdrCz, Observation/Observation-TravelHistory-Madagaskar, Patient/48a9d440-4194-42c1-87ad-b5a39020a4d0, Patient/Mracena2, PatientHistory2FHIRCzHdr, PlanOfCare2FHIRCzHdr, Practitioner/Practitioner-L1, RelatedPersonRelationshipTypesHDR and TemporaryHDRSystem
- Produced by HL7 under the terms of HL7® Governance and Operations Manual relating to Intellectual Property (Section 16), specifically its copyright, trademark and patent provisions. This document is licensed under Creative Commons "No Rights Reserved" (CC0).
Show Usage
- The UCUM codes, UCUM table (regardless of format), and UCUM Specification are copyright 1999-2009, Regenstrief Institute, Inc. and the Unified Codes for Units of Measures (UCUM) Organization. All rights reserved. https://ucum.org/trac/wiki/TermsOfUse
Show Usage
- Unified Code for Units of Measure (UCUM): Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections... Show 19 more, Medication/Medication-Euthyrox, Medication/med-CovidVaccineComirnaty, Medication/med-Ibalgin400, Medication/med-paracetamol, Observation/10f5c49e-086d-4016-8dd1-10000000001b, Observation/4ba395b7-be9e-4bed-bef7-1c8f0b2d4e3a, Observation/5c2ddf62-9785-493f-80c6-8b0d1e3a4b2c, Observation/5c363e2d-c4e1-436d-bad7-0b3f8c6a9f1d, Observation/6bec5d97-a17e-4015-8fce-7b1c0c3a2f4b, Observation/6c626338-82ba-46a1-bcb8-2c8f0b2d4e3a, Observation/7cf304de-5ae3-4621-8531-9c8f0b2d4e3a, Observation/8d2aea77-f576-4d0f-9508-537359aa44d6, Observation/GynNaturalBirth-BirthLength, Observation/GynNaturalBirth-BirthWeight, Observation/hb-result, Observation/hct-result, Observation/plt-result, Observation/rbc-result and Observation/wbc-result
- These codes are excerpted from ASTM Standard, E1762-95(2013) - Standard Guide for Electronic Authentication of Health Care Information, Copyright by ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA 19428. Copies of this standard are available through the ASTM Web Site at www.astm.org.
Show Usage
- This material contains content from LOINC. LOINC is copyright © 1995-2020, Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC) Committee and is available at no cost under the license. LOINC® is a registered United States trademark of Regenstrief Institute, Inc.
Show Usage
- LOINC: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 51 more, Bundle/DischargeBundle-Novak-Petr-L1-core, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CZConsentCategory, CZ_CompositionHdr, CZ_CompositionObligationsHdr, CZ_ConsentHdr, CZ_ImmunizationRecommendationHdr, CZ_ImmunizationRecommendationObligationsHdr, CZ_ObservationAbdominalCircumferenceHdr, CZ_ObservationBMIHdr, CZ_ObservationBirthLength, CZ_ObservationBirthWeight, CZ_ObservationChestCircumferenceHdr, CZ_ObservationGravidity, CZ_ObservationHeadCircumferenceHdr, CZ_ObservationHeightHdr, CZ_ObservationMultiplePregnancy, CZ_ObservationParity, CZ_ObservationSdohHdr, CZ_ObservationTravelHdr, CZ_ObservationWeightHdr, Composition/6891fd68-dc3c-4c91-a8d3-cb5ec990c035, Composition/6891fd68-dc3c-4c91-a8d3-cb5ec990c03b, Composition/6e9f3ec7-0a3c-4ee4-a2f2-a9c6ad91d001, Composition/701f51d5-78bf-428e-a6b5-349c2614ce07, Composition/cdae7735-f7ee-4bc7-9cf3-3dc806a4eaaf, DocumentReference/66678621-df93-47ca-a36c-2a39a92472e7, DocumentReference/d9ad8a22-a12b-48db-938c-4066a3a0617a, ImmunizationRecommendation/ImmunizationRecommendation, Observation/10f5c49e-086d-4016-8dd1-10000000001b, Observation/3f85726c-ad2f-441b-89ce-100000000021, Observation/4ba395b7-be9e-4bed-bef7-1c8f0b2d4e3a, Observation/5c2ddf62-9785-493f-80c6-8b0d1e3a4b2c, Observation/5c363e2d-c4e1-436d-bad7-0b3f8c6a9f1d, Observation/6bec5d97-a17e-4015-8fce-7b1c0c3a2f4b, Observation/6c626338-82ba-46a1-bcb8-2c8f0b2d4e3a, Observation/7cf304de-5ae3-4621-8531-9c8f0b2d4e3a, Observation/8d2aea77-f576-4d0f-9508-537359aa44d6, Observation/CZObservationSdohHdrExample, Observation/GynNaturalBirth-Apgar1, Observation/GynNaturalBirth-Apgar10, Observation/GynNaturalBirth-Apgar5, Observation/GynNaturalBirth-BirthLength, Observation/GynNaturalBirth-BirthWeight, Observation/GynNaturalBirth-FetalPresentation, Observation/GynNaturalBirth-GestationalAgeAtDelivery, Observation/GynNaturalBirth-Gravidity, Observation/GynNaturalBirth-MultiplePregnancy, Observation/GynNaturalBirth-Parity and Observation/Observation-TravelHistory-Madagaskar
- This material contains content that is copyright of SNOMED International. Implementers of these specifications must have the appropriate SNOMED CT Affiliate license - for more information contact https://www.snomed.org/get-snomed or info@snomed.org.
Show Usage
- SNOMED Clinical Terms® (SNOMED CT®): AllergyIntolerance/6cf80cb1-9766-470f-ac36-b1d3d8950f1b, AllergyIntolerance/AllergyInfoUnknown... Show 75 more, AllergyIntolerance/MilkAllergy, AllergyIntolerance/NoKnownAllergy, AllergyIntolerance/PenicillinAllergy, AllergyIntolerance/StrawberryAllergy, Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1, Bundle/DischargeBundle-Novak-Petr-L1-core, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CZProvisionCode, CZ_CompositionHdr, CZ_CompositionObligationsHdr, CZ_ConditionHdr, CZ_DeviceUseStatementHdr, CZ_EncounterHdr, CZ_FamilyMemberHistoryHdr, CZ_ImmunizationHdr, CZ_ImmunizationRecommendationHdr, CZ_ObservationInfectiousContactHdr, CZ_ObservationSdohHdr, CZ_ProcedureHdr, Composition/701f51d5-78bf-428e-a6b5-349c2614ce07, Condition/35717696-8a99-4f99-a938-ec0ec88a65a2, Condition/AngiodysplasiaCondition, Condition/AtrialFibrillationCondition, Condition/CZ-Condition-HDR-Example, Condition/CZ-Condition-HDR-Example-2, Condition/CZ-Condition-HDR-Example-3, Condition/HypercholesterolemiaCondition, Condition/HypothyroidismCondition, Condition/IronDeficiencyAnemiaCondition, Condition/OsteoporosisCondition, Condition/PresbycusisCondition, Condition/TIACondition, Condition/VaricoseVeinsCondition, Condition/fdf9e92d-ac48-4706-b15b-d2eaca85f45f, Device/Device-Pacemaker, DeviceUseStatement/DeviceUseStatement-Pacemaker, EHDSIConditionPOA, EHDSITreatmentClass, Encounter/CZ-Encounter-HDR-Example, ExposureAgentHdrVs, Goal/620b1120-cece-44b1-89f5-20413054eb1d, Immunization/3f85726c-ad2f-441b-89ce-10000000001e, Immunization/Immunization-CovidExample, ImmunizationRecommendation/ImmunizationRecommendation, MedicationStatement/47472c99-09bf-4007-bfaa-16c9665ae090, MedicationStatement/bf08b62b-0abd-4e88-9092-ce0228382e51, MedicationStatement/f34114fc-138f-4bd8-8e1a-804d14ec9986, Observation/3f85726c-ad2f-441b-89ce-10000000001c, Observation/3f85726c-ad2f-441b-89ce-10000000001d, Observation/3f85726c-ad2f-441b-89ce-100000000021, Observation/3f85726c-ad2f-441b-89ce-100000000022, Observation/3f85726c-ad2f-441b-89ce-100000000023, Observation/3f85726c-ad2f-441b-89ce-100000000024, Observation/3f85726c-ad2f-441b-89ce-100000000025, Observation/3f85726c-ad2f-441b-89ce-100000000027, Observation/CZObservationSdohHdrExample, Observation/ExampleSdohAlcohol, Observation/ExampleSdohSmoking, Observation/GynNaturalBirth-FetalPresentation, Observation/Observation-Education-level, Observation/e15aeeaf-e288-404c-9704-9c8f0b2d4e3a, PractitionerRole/2b7e9637-5018-4542-9faf-d5abdee7b849, PractitionerRole/69d34ceb-b556-4f75-9e4c-9184fe8a10c5, PractitionerRole/Practitioner-Referrer-detail, PractitionerRole/practitionerrole1, Procedure/CZ-Procedure-HDR-Example-Acute-appendicitis, Procedure/ColonoscopyProcedure, Procedure/GynNaturalBirth-DeliveryMethod, Procedure/Procedure-Appendectomy, Procedure/Procedure-Insert-Pacemaker, Procedure/Procedure-Insert-Pacemaker2, Procedure/ffb1a62f-9050-4e33-af4b-4cdb8203c9e5 and Specimen/Specimen-EDTA-1
- This material derives from the HL7 Terminology (THO). THO is copyright ©1989+ Health Level Seven International and is made available under the CC0 designation. For more licensing information see: https://terminology.hl7.org/license.html
Show Usage
- Admit source: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 4 more, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, Encounter/10f5c49e-086d-4016-8dd1-b555306bf620 and Encounter/CZ-Encounter-HDR-Example
- AllergyIntolerance Clinical Status Codes: AllergyIntolerance/6cf80cb1-9766-470f-ac36-b1d3d8950f1b, AllergyIntolerance/AllergyInfoUnknown... Show 6 more, AllergyIntolerance/MilkAllergy, AllergyIntolerance/NoKnownAllergy, AllergyIntolerance/PenicillinAllergy, AllergyIntolerance/StrawberryAllergy, Bundle/DischargeBundle-Novak-Petr and Bundle/DischargeBundle-Novak-Petr-Subsections
- AllergyIntolerance Verification Status: AllergyIntolerance/6cf80cb1-9766-470f-ac36-b1d3d8950f1b, AllergyIntolerance/MilkAllergy... Show 5 more, AllergyIntolerance/NoKnownAllergy, AllergyIntolerance/PenicillinAllergy, AllergyIntolerance/StrawberryAllergy, Bundle/DischargeBundle-Novak-Petr and Bundle/DischargeBundle-Novak-Petr-Subsections
- Condition Category Codes: Condition/AngiodysplasiaCondition, Condition/AtrialFibrillationCondition... Show 8 more, Condition/CZ-Condition-HDR-Example, Condition/HypercholesterolemiaCondition, Condition/HypothyroidismCondition, Condition/IronDeficiencyAnemiaCondition, Condition/OsteoporosisCondition, Condition/PresbycusisCondition, Condition/TIACondition and Condition/VaricoseVeinsCondition
- Condition Clinical Status Codes: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections... Show 15 more, CZ_ConditionHdr, Condition/35717696-8a99-4f99-a938-ec0ec88a65a2, Condition/AngiodysplasiaCondition, Condition/AtrialFibrillationCondition, Condition/CZ-Condition-HDR-Example, Condition/CZ-Condition-HDR-Example-2, Condition/CZ-Condition-HDR-Example-3, Condition/HypercholesterolemiaCondition, Condition/HypothyroidismCondition, Condition/IronDeficiencyAnemiaCondition, Condition/OsteoporosisCondition, Condition/PresbycusisCondition, Condition/TIACondition, Condition/VaricoseVeinsCondition and Condition/fdf9e92d-ac48-4706-b15b-d2eaca85f45f
- ConditionVerificationStatus: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections... Show 15 more, CZ_ConditionHdr, Condition/35717696-8a99-4f99-a938-ec0ec88a65a2, Condition/AngiodysplasiaCondition, Condition/AtrialFibrillationCondition, Condition/CZ-Condition-HDR-Example, Condition/CZ-Condition-HDR-Example-2, Condition/CZ-Condition-HDR-Example-3, Condition/HypercholesterolemiaCondition, Condition/HypothyroidismCondition, Condition/IronDeficiencyAnemiaCondition, Condition/OsteoporosisCondition, Condition/PresbycusisCondition, Condition/TIACondition, Condition/VaricoseVeinsCondition and Condition/fdf9e92d-ac48-4706-b15b-d2eaca85f45f
- Consent Category Codes: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections, Consent/CZ-AdvanceDirectives-HDR-CORE and Consent/CZ-AdvanceDirectives-HDR-DNR
- Consent Scope Codes: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections, Consent/CZ-AdvanceDirectives-HDR-CORE and Consent/CZ-AdvanceDirectives-HDR-DNR
- Diagnosis Role: Encounter/CZ-Encounter-HDR-Example
- Discharge disposition: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 4 more, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, Encounter/10f5c49e-086d-4016-8dd1-b555306bf620 and Encounter/CZ-Encounter-HDR-Example
- Immunization Recommendation Status Codes: ImmunizationRecommendation/ImmunizationRecommendation
- Location type: Nemocnice Chrudim - Oddělení CHIR-JIP, Nemocnice Chrudim - Oddělení CHIR1... Show 5 more, Nemocnice Praha, Nemocnice Praha - Klinika Chirurgie, Nemocnice Praha - Oddělení A, Nemocnice Praha - Oddělení B and Nemocnice Praha - Oddělení C
- Medication request administration location codes: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections, MedicationStatement/47472c99-09bf-4007-bfaa-16c9665ae090, MedicationStatement/bf08b62b-0abd-4e88-9092-ce0228382e51 and MedicationStatement/f34114fc-138f-4bd8-8e1a-804d14ec9986
- Observation Category Codes: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections... Show 22 more, CZ_ObservationSdohHdr, Observation/10f5c49e-086d-4016-8dd1-10000000001b, Observation/3f85726c-ad2f-441b-89ce-10000000001c, Observation/3f85726c-ad2f-441b-89ce-10000000001d, Observation/3f85726c-ad2f-441b-89ce-100000000021, Observation/3f85726c-ad2f-441b-89ce-100000000022, Observation/3f85726c-ad2f-441b-89ce-100000000023, Observation/3f85726c-ad2f-441b-89ce-100000000024, Observation/3f85726c-ad2f-441b-89ce-100000000025, Observation/3f85726c-ad2f-441b-89ce-100000000027, Observation/4ba395b7-be9e-4bed-bef7-1c8f0b2d4e3a, Observation/5c2ddf62-9785-493f-80c6-8b0d1e3a4b2c, Observation/5c363e2d-c4e1-436d-bad7-0b3f8c6a9f1d, Observation/6bec5d97-a17e-4015-8fce-7b1c0c3a2f4b, Observation/6c626338-82ba-46a1-bcb8-2c8f0b2d4e3a, Observation/7cf304de-5ae3-4621-8531-9c8f0b2d4e3a, Observation/8d2aea77-f576-4d0f-9508-537359aa44d6, Observation/CZObservationSdohHdrExample, Observation/ExampleSdohAlcohol, Observation/ExampleSdohSmoking, Observation/Observation-Education-level and Observation/e15aeeaf-e288-404c-9704-9c8f0b2d4e3a
- contactRole2: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 4 more, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, Patient/3f85726c-ad2f-441b-89ce-100000000000 and Patient/Mracena2
- identifierType: CZ_PatientObligationsHdr, CZ_RelatedPersonHdr, CZ_RelatedPersonObligationsHdr and Patient/Mracena2
- providerRole: CZ_ImmunizationHdr and CZ_ImmunizationObligationsHdr
- ActClass: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections, CZ_ObservationInfectiousContactHdr and Observation/Observation-InfectiousContact
- ActCode: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 8 more, Bundle/DischargeBundle-Novak-Petr-L1-core, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CZ_EncounterHdr, Encounter/10f5c49e-086d-4016-8dd1-b555306bf620, Encounter/CZ-Encounter-HDR-Example, Encounter/HospitalEncounter and Encounter/f08151d0-a7ad-4a7b-b7b9-97eb1d394ffb
- ActPriority: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 5 more, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CZ_EncounterHdr, Encounter/10f5c49e-086d-4016-8dd1-b555306bf620 and Encounter/CZ-Encounter-HDR-Example
- Education Level: Observation/Observation-Education-level
- ObservationInterpretation: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-Subsections... Show 7 more, Observation/10f5c49e-086d-4016-8dd1-10000000001b, Observation/3f85726c-ad2f-441b-89ce-100000000021, Observation/hb-result, Observation/hct-result, Observation/plt-result, Observation/rbc-result and Observation/wbc-result
- ParticipationType: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 10 more, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CZ_EncounterHdr, CZ_EncounterObligationsHdr, CZ_ObservationInfectiousContactHdr, Encounter/10f5c49e-086d-4016-8dd1-b555306bf620, Encounter/CZ-Encounter-HDR-Example, Observation/Observation-InfectiousContact, Provenance/cdae7735-f7ee-4bc7-9cf3-3dc806a4eaab and Provenance/cdae7735-f7ee-4bc7-9cf3-3dc806a4eabb
- RoleCode: Bundle/DischargeBundle-Novak-Petr, Bundle/DischargeBundle-Novak-Petr-L1... Show 6 more, Bundle/DischargeBundle-Novak-Petr-L1-plus, Bundle/DischargeBundle-Novak-Petr-Subsections, CZ_RelatedPersonHdr, Patient/3f85726c-ad2f-441b-89ce-100000000000, Patient/Mracena2 and RelatedPersonRelationshipTypesHDR
