HL7 FHIR Implementation Guide: Common Terminology Criteria (CTC) Adverse Events Release 1 - DRAFT
0.0.1 - DRAFT
HL7 FHIR Implementation Guide: Common Terminology Criteria (CTC) Adverse Events Release 1 - DRAFT
0.0.1 - DRAFT
HL7 FHIR Implementation Guide: Common Terminology Criteria (CTC) Adverse Events Release 1 - DRAFT, published by HL7 International Clinical Interoperability Council. This guide is not an authorized publication; it is the continuous build for version 0.0.1 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/standardhealth/fsh-ae/ and changes regularly. See the Directory of published versions
| Official URL: http://hl7.org/fhir/us/ctcae/StructureDefinition/adverse-event-expectation | Version: 0.0.1 | |||
| Draft as of 2024-02-28 | Computable Name: AdverseEventExpectation | |||
A determination if the adverse event is or is not one whose nature and severity have been previously observed, identified in nature, severity, or frequency, and documented in the investigator brochure, investigational plan, protocol, current consent form, scientific publication, or in other relevant and reliable document.
Context of Use
This extension may be used on the following element(s):
Usage info
Usage:
Description of Profiles, Differentials, Snapshots, and how the XML and JSON presentations work.
This structure is derived from Extension
Summary
Simple Extension of type CodeableConcept: A determination if the adverse event is or is not one whose nature and severity have been previously observed, identified in nature, severity, or frequency, and documented in the investigator brochure, investigational plan, protocol, current consent form, scientific publication, or in other relevant and reliable document.
This structure is derived from Extension
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Extension | Adverse Event Expectation | |
  | 0..0 | |||
  | 1..1 | uri | "http://hl7.org/fhir/us/ctcae/StructureDefinition/adverse-event-expectation" | |
 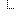 | 0..1 | CodeableConcept | Value of extension Binding: Adverse Event Expectation Value Set (required) | |
 Documentation for this format Documentation for this format | ||||
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Extension | Adverse Event Expectation | |
  | 0..1 | string | Unique id for inter-element referencing | |
  | 0..0 | |||
  | 1..1 | uri | "http://hl7.org/fhir/us/ctcae/StructureDefinition/adverse-event-expectation" | |
 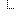 | 0..1 | CodeableConcept | Value of extension Binding: Adverse Event Expectation Value Set (required) | |
 Documentation for this format Documentation for this format | ||||
This structure is derived from Extension
Summary
Simple Extension of type CodeableConcept: A determination if the adverse event is or is not one whose nature and severity have been previously observed, identified in nature, severity, or frequency, and documented in the investigator brochure, investigational plan, protocol, current consent form, scientific publication, or in other relevant and reliable document.
Differential View
This structure is derived from Extension
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Extension | Adverse Event Expectation | |
  | 0..0 | |||
  | 1..1 | uri | "http://hl7.org/fhir/us/ctcae/StructureDefinition/adverse-event-expectation" | |
 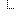 | 0..1 | CodeableConcept | Value of extension Binding: Adverse Event Expectation Value Set (required) | |
 Documentation for this format Documentation for this format | ||||
Snapshot View
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Extension | Adverse Event Expectation | |
  | 0..1 | string | Unique id for inter-element referencing | |
  | 0..0 | |||
  | 1..1 | uri | "http://hl7.org/fhir/us/ctcae/StructureDefinition/adverse-event-expectation" | |
 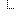 | 0..1 | CodeableConcept | Value of extension Binding: Adverse Event Expectation Value Set (required) | |
 Documentation for this format Documentation for this format | ||||
Other representations of profile: CSV, Excel, Schematron
| Path | Conformance | ValueSet | URI |
| Extension.value[x] | required | AdverseEventExpectationVShttp://hl7.org/fhir/us/ctcae/ValueSet/adverse-event-expectation-value-setfrom this IG |