HL7 FHIR Implementation Guide: Common Terminology Criteria (CTC) Adverse Events Release 1 - DRAFT
0.0.1 - DRAFT
HL7 FHIR Implementation Guide: Common Terminology Criteria (CTC) Adverse Events Release 1 - DRAFT
0.0.1 - DRAFT
HL7 FHIR Implementation Guide: Common Terminology Criteria (CTC) Adverse Events Release 1 - DRAFT, published by HL7 International Clinical Interoperability Council. This guide is not an authorized publication; it is the continuous build for version 0.0.1 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/standardhealth/fsh-ae/ and changes regularly. See the Directory of published versions
| Official URL: http://hl7.org/fhir/us/ctcae/ImplementationGuide/hl7.fhir.us.ctcae | Version: 0.0.1 | |||
| Draft as of 2024-02-28 | Computable Name: HL7FHIRImplementationGuideCTCAdverseEventsRelease1USRealmSTU1 | |||
NOTE: This is a very early and preliminary version of the AdverseEvent specification. Please do not submit comments on the IG content at this time.
Adverse event (AE) reporting is necessary to document any unfavorable and unintended sign, symptom or disease that occurs in a patient who is undergoing a medical treatment or procedure. The Common Terminology Criteria for Adverse Events (CTCAE) are a set of criteria for the standardized classification of adverse effects of drugs used in cancer therapy. The CTCAE 5.0 terminology will be used for this Implementation Guide. CTCAE are a set of criteria for the standardized classification of adverse effects of drugs used in cancer therapy. Each CTCAE term is mapped to a Medical Dictionary for Regulatory Activities (MedDRA) term and code, and many clinical trials encode their observations based on the CTCAE system.
The collection of adverse events differs across institutions and there are separate workflows for the capture of adverse events in clinical trials depending on the role. Clinical trial coordinators oftentimes abstract or transcribe relevant elements for trials reporting. In many cases, this results in duplicative efforts in a form that is not easily exchangeable.
It is currently not possible to assess treatment-associated adverse events reliably using electronic health record (EHR) data. Advantages of properly structured EHR data include creating a single data input system, which maximizes efficiency, reduces cost, and eliminates errors associated with double data entry and transcriptions from one data collection system to another.
The CTC Adverse Event is a minimal common oncology data elements (mCODE) compatible structured data model to support embedding CTCAE reporting within the EHR.
The information obtained from subject matter experts, pre-existing standards, nomenclatures, and guidelines were consulted in the development of this specification, including:
Other reporting standards informing the adverse event work:
Several projects related to Adverse Event reporting are underway at HL7, including:
We recognize the need to have a more cohesive effort to create an overarching and collaborative FHIR model that could represent these use cases. While collaborative discussions are underway, this IG distinguishes itself in several ways:
There are multiple actors recognized in this IG including:
One use case currently driving this IG is the Integrating Clinical Trials and Real-world Endpoints data (ICAREdata) initiative sponsored by the Alliance for Clinical Trials in Oncology. It aims to overcome the challenges of using the EHR as a method of collecting high quality clinical trials data by developing data structures and data collection methods that accurately report clinical trials data while effectively addressing provider burden. The approach to testing ICAREdata methods will also be used for adverse event reporting. The ICAREdata Adverse Event Reporting use case will test this mCODE-compatible structured data model for the CTCAE.
The ICAREdata pilot environment is likely representative of multiple environments seeking to better integrate adverse event data captured from an electronic health record (EHR) with that of a dedicated reporting system for reporting adverse events to a monitoring organization like CTEP.
The systems considered in this IG include:
The diagram below shows one example where several Alliance for Clinical Trials (ACT) sites could use one or more forms used for the capture of adverse events.
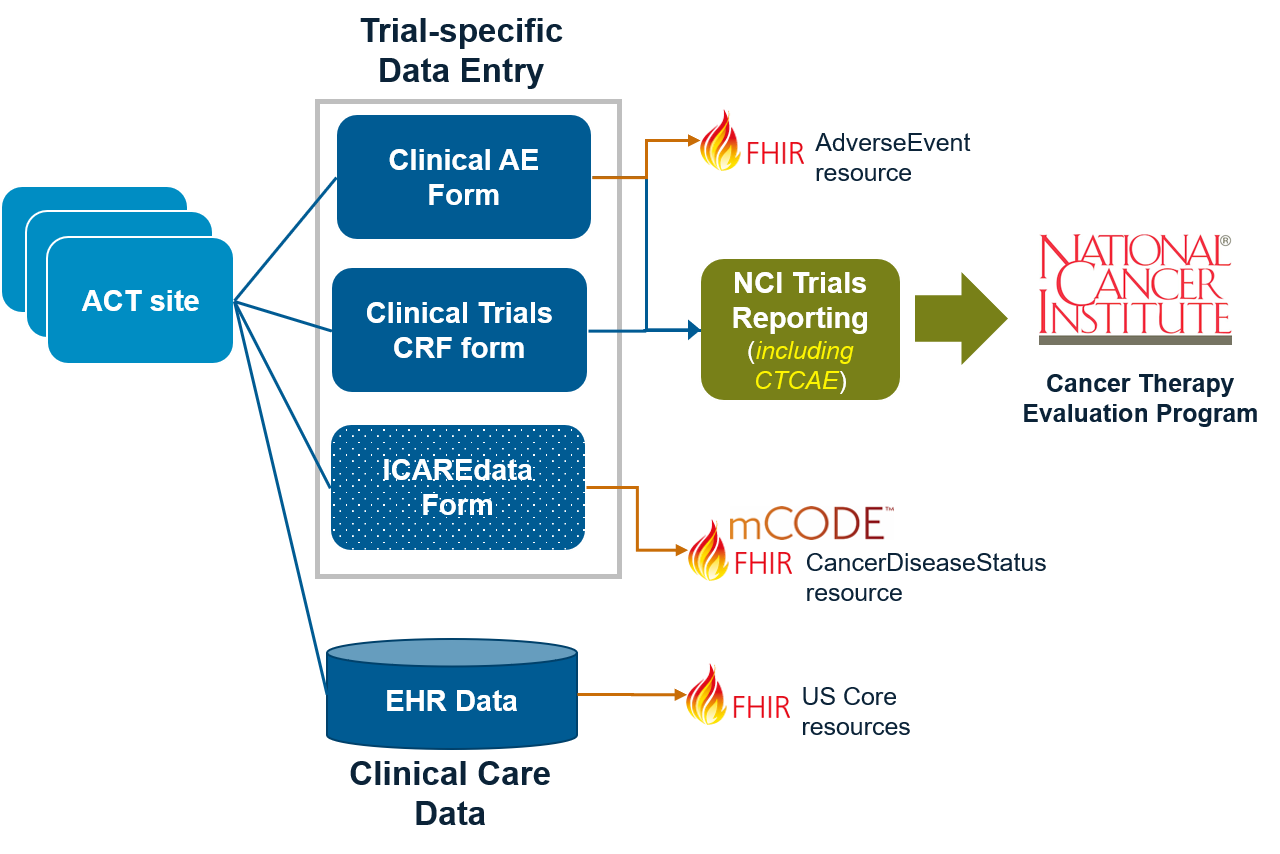
The following use cases will inform the initial design for this IG:
Use Case 1: A clinical research coordinator or principal investigator submits an electronic Case Report Form for clinical trial patient receiving a treatment and experiences one or more adverse events (AE).
Use Case 2: A patient experiences an adverse event during or after the administration a given drug, vaccine, or biological product (e.g.: drug transfusion). The provider records the adverse event per healthcare organization patient safety policies.
This implementation guide (IG) is a Domain of Knowledge IG. The purpose of this IG is to show how to represent clinical concepts generally, not to have a complete set of agreements for interoperable exchanges.
The authors recognize the leadership and sponsorship of Dr. Monica Bertagnolli and Dr. Jay Schnitzer, MITRE Chief Technology Officer. Dr. Steven Piantadosi and the Alliance for Clinical Trials in Oncology coordinated real-world data collection in clinical trials, as part of this project. Lead MITRE contributors were Mark Kramer, Ph.D, and May Terry, RN. Andre Quina and Dr. Brian Anderson guide the overall mCODE and CodeX efforts at MITRE.
This IG was authored by the MITRE Corporation using FHIR Shorthand (FSH) and SUSHI, a free, open source toolchain from MITRE Corporation.
| Co-Editor: | Michelle Casagni MITRE Corporation mcasagni@mitre.org |
| Co-Editor: | Mark Kramer MITRE Corporation mkramer@mitre.org |
| Co-Editor: | May Terry MITRE Corporation mayt@mitre.org |
MITRE: Approved for Public Release. Distribution Unlimited. Case Number 16-1988