NHS North West Genomics
0.2.1 - ci-build
NHS North West Genomics
0.2.1 - ci-build
NHS North West Genomics, published by NHS North West Genomics. This guide is not an authorized publication; it is the continuous build for version 0.2.1 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/nw-gmsa/nw-gmsa.github.com/ and changes regularly. See the Directory of published versions
| Official URL: https://fhir.nwgenomics.nhs.uk/ImplementationGuide/fhir.nwgenomics.nhs.uk | Version: 0.2.1 | |||
| Draft as of 2026-02-27 | Computable Name: NWGMSA | |||
Diagnostic testing is essential to modern clinical care, offering objective information that supports decision-making at every stage of a patient’s journey—from initial evaluation to long-term monitoring and assessment of outcomes.
Genomic diagnostic testing contributes to this process by examining a patient’s DNA or RNA to detect genetic variations that influence disease susceptibility, diagnosis, treatment choices, and prognosis. By delivering highly specific and personalised insights, genomic testing improves the accuracy and effectiveness of clinical management.
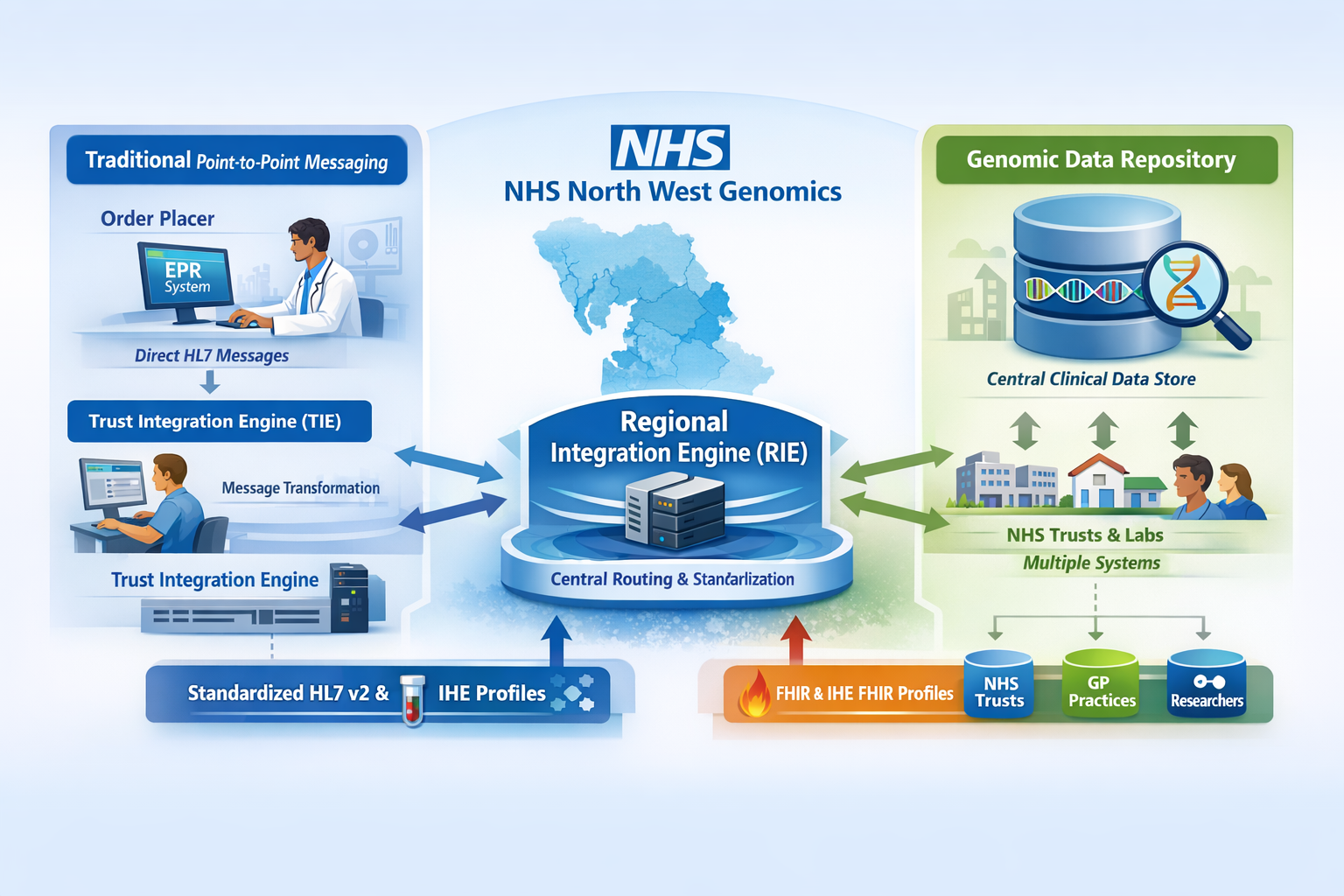
NHS North West Genomics is a new regional NHS service that consolidates clinical genomic testing across the North West of England. Although the service is delivered regionally, it also processes genomic test requests from across the UK. The service is hosted by Manchester University NHS Foundation Trust.
As part of the service transition, existing systems for electronic test ordering and reporting will be enhanced through the introduction of a Regional Integration Engine (RIE) and a Genomic Clinical Data Repository. These components enable seamless data exchange between local clinical systems and regional genomic laboratory services.
Although NW GMSA is hosted by Manchester University NHS Foundation Trust, it operates in practice as a distinct organisation. It has its own Trust Integration Engine (TIE), referred to as the Regional Integration Engine (RIE).
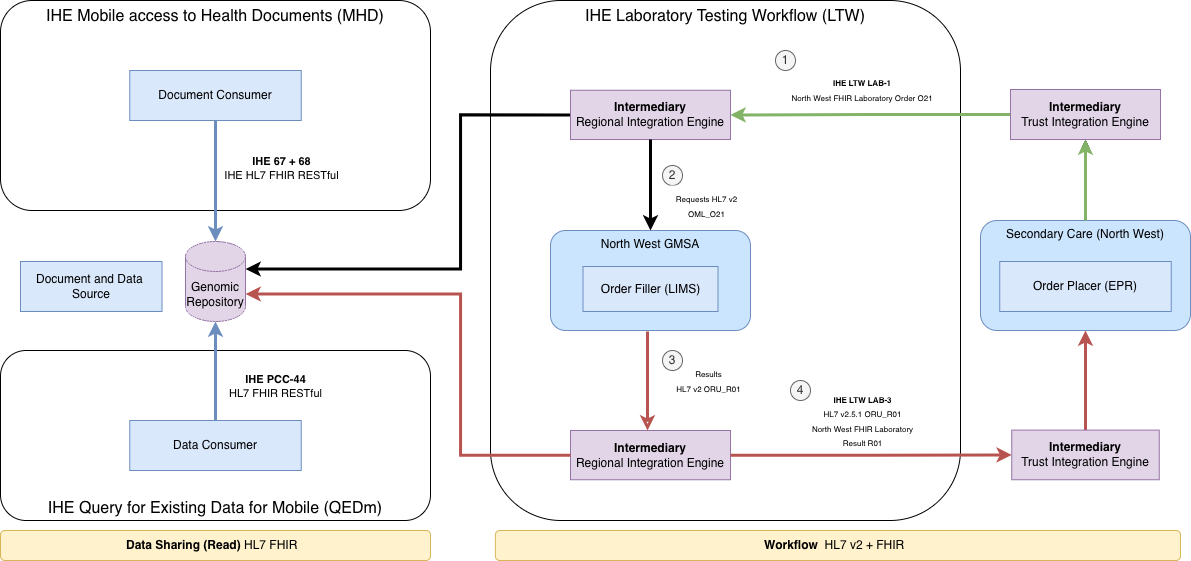
At present, LIMS and EPR systems across the North West use a range of HL7 v2 Message based workflows, this remains the case with the RIE (centre and right of the above diagram). To reduce this variation, the IHE Laboratory Testing Workflow (LTW) profile and region-wide genomic messaging standards (HL7 v2.5.1 and FHIR R4) have been adopted. This standardisation applies to interactions between the NW GMSA Regional Integration Engine (RIE) and NHS Trust Integration Engines (TIEs). Interactions between LIMS and EPR systems, as well as the internal integration engine configurations within trusts, remain unchanged.
For external systems and NHS Trusts, NW Genomics LIMS will present as a single system with unified ordering and reporting interfaces. Direct point-to-point integrations between individual NHS Trust EPR systems and the NW LIMS will not be supported.
The NW Genomics HL7 v2 + FHIR specifications and the IHE LTW standards are detailed in this Implementation Guide.
In addition, NW Genomics is delivering a Genomic Clinical Repository (GCR) to support broader sharing of genomic test results across NHS Trusts, Integrated Care Systems (ICSs), and other Genomic Medicine Service Alliances (GMSAs). This GCR is populated by 'wire-taps' on existing workflows and solely HL7 FHIR R4 based plus IHE QEDm and MHD profiles to provide an additional layer of standardisation.
Genomic Document Sharing is a proposed new capability within NW Genomics that leverages the existing NHS England National Record Locator (NRL). The solution is aligned with the IHE Mobile Health Document Sharing (MHDS) profile and so, follows a similar architectural approach to that used by the NHS England National Imaging Registry in its integration with the NRL.
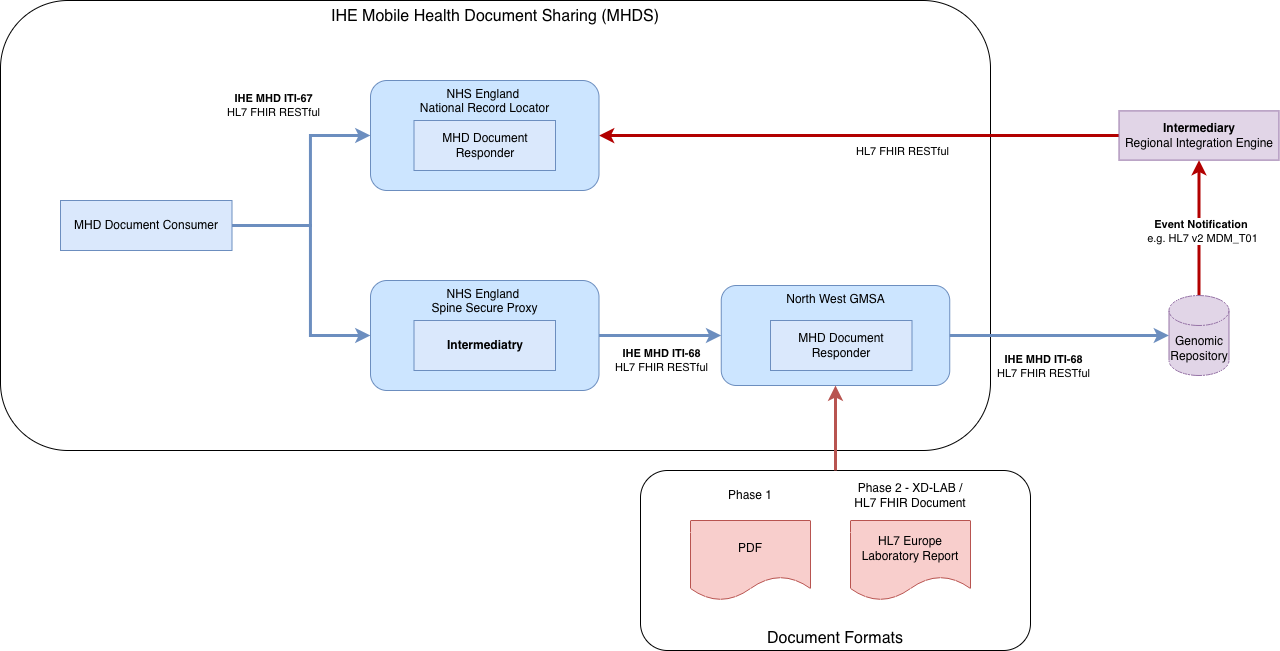
For the initial phase, genomic reports will be published in PDF format. In later phases, the target format is the HL7 Europe Laboratory Report, aligning with the (anticipated) strategic direction for NHS England Pathology FHIR, which specifies FHIR-based pathology reporting.
| Menu Item | Description | Audience | |
|---|---|---|---|
| Analysis and Design (Volume 1) | Description of the processes and corresponding technical frameworks | General | |
| Interfaces (Volume 2) | Description of the processes and corresponding technical frameworks (HL7 v2 and FHIR Interactions) | Detailed Technical (Integration Developer) | |
| Data Models (Volume 3) | NHS North West Forms, Templates, Reports, and Compositions | Data Modeling (Detailed Technical) | |
| Artefacts (Volume 4) | NHS North West Common Data Models | Detailed Technical | |
| Development | Testing, Suppport and Architecture | Detailed Technical (Developer) |
UK edition of SNOMED (83821000000107)
Package hl7.fhir.uv.extensions.r4#5.2.0 This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Mon, Feb 10, 2025 21:45+1100+11:00) |
Package hl7.fhir.uv.ipa#1.1.0 This IG describes how an application acting on behalf of a patient can access information about the patient from an clinical records system using a FHIR based API. The clinical records system may be supporting a clinical care provider (e.g. a hospital, or a general practitioner), or a health data exchange, including a national health record system. (built Wed, Mar 19, 2025 14:34+0000+00:00) |
Package hl7.fhir.uv.xver-r5.r4#0.0.1-snapshot-2 The cross-version extensions available in FHIR R4 from FHIR R5 (built Sat, Sep 13, 2025 16:55-0400-04:00) |
Package hl7.fhir.eu.extensions.r4#1.2.0 This guide lists the extensions specified for the European REALM. (built Fri, Dec 19, 2025 11:52+0100+01:00) |
Package ihe.pharm.mpd.r4#1.0.0-comment-2 ImplementationGuide for IHE Pharmacy Medication Prescription and Dispense (MPD) profile (built Tue, May 27, 2025 16:32+0200+02:00) |
Package hl7.fhir.eu.base#2.0.0-ballot This guide collects base and core profiles to be used in the European context. It also includes common artifacts, such as the profiles describing the European Health Insurance Card. (built Fri, Dec 19, 2025 18:28+0100+01:00) |
Package fhir.r4.ukcore.stu3.currentbuild#0.0.19-pre-release UK Core FHIR profiles and Assets |
Package hl7.fhir.uv.extensions.r4#5.1.0 This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Sat, Apr 27, 2024 18:39+1000+10:00) |
Package hl7.fhir.uv.genomics-reporting#3.0.0 Guidelines for reporting of clinical genomics results using HL7 FHIR. (built Thu, Dec 12, 2024 20:34+0000+00:00) |
Package hl7.fhir.uv.ips#1.1.0 International Patient Summary (IPS) FHIR Implementation Guide (built Tue, Nov 22, 2022 03:24+0000+00:00) |
Package hl7.fhir.eu.laboratory#0.1.1 This guide describes how the Laboratory Report can be represented in the European REALM. (built Tue, Mar 25, 2025 12:00+0100+01:00) |
Package hl7.fhir.uv.sdc#3.0.0 The SDC specification provides an infrastructure to standardize the capture and expanded use of patient-level data collected within an EHR. |
Package hl7.fhir.uv.tools.r4#0.9.0 This IG defines the extensions that the tools use internally. Some of these extensions are content that are being evaluated for elevation into the main spec, and others are tooling concerns (built Tue, Dec 16, 2025 23:18+1100+11:00) |
| Role(s) | Contributor(s) |
|---|---|
| North West Genomic Medicine Service Alliance | |
| Alder Hey Children's Hospital Trust | |
| Manchester University NHS Foundation Trust | |
| Liverpool Womens NHS Foundation Trust | |
| The Christie NHS Foundation Trust | |
| NHS England | |
| Staff Engineer | Kevin Mayfield, Aire Logic & Mayfield IS |