HL7 Europe Imaging Study Report, published by HL7 Europe. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/hl7-eu/imaging/ and changes regularly. See the Directory of published versions
This model is (still) based on a logical model based on the eHN Imaging Studies and Reports. It will need to be updated to the XtEHR Imaging Logical Model
|
|
Update the model |
The European eHealth Network "Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU Medical imaging studies and reports, release 1.1" is addressed to the Member States of the European Union and applies to the implementation of interoperable cross-border exchange of imaging study reports in order to support safe and efficient provisioning of care services in another member state.
It could also serve as a guiding principle for the national development and implementation of imaging study reports.
The eHN Imaging Studies and Reports guidelines in section 4 specifies a DATASET, which is a simplified logical model of a imaging study manifest and report. The data set comprises of several basic parts as visualized in the diagram below.
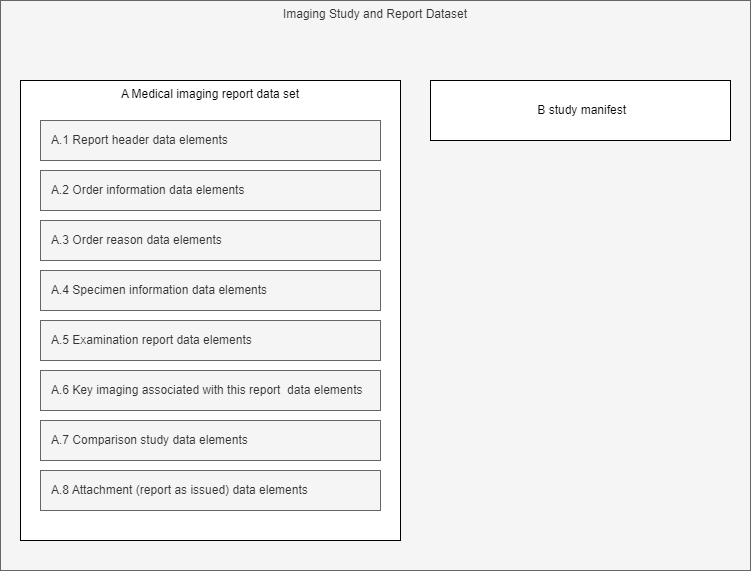
The following table lists the HL7 FHIR logical models used to represent this dataset. To facilitate the references with the eHN Imaging Studies and Reports guidelines data sets the short description of each element reports the label of the eHN element (e.g., A.1.7.2 Result validator name).
The HL7 FHIR logical model requires that element cardinality is specified, while the eHN Imaging Studies and Reports guidelines data set doesn't define them on purpose. For this reason the elements' cardinality of the following FHIR Logical Model should be interpreted with this in mind, thus they should not be considered as "normative".
| Title | Description |
| B Imaging study manifest data set (eHN) | The data set defines the contents of the key information about the imaging study as conveyed by the imaging study manifest data set. The imaging study manifest contains key information about the imaging study that is referenced, including the “pointers” that allow access to the series of images. It is important to note that the metadata used in expressing the filters associated with the querying for a list of imaging studies and/or imaging reports are defined in Section 2 Article 10: Selection List and filtering Parameters. These parameters are expressed as coded values from standardised value sets to ensure a robust search for a list of relevant imaging studies. Such metadata filtering parameters are associated with imaging studies, but may not be present in the content of the imaging study manifest. |
| A Medical Imaging Report (eHN) | Medical Imaging Report |
| A.1.5 Author | Author (by whom the imaging report or a subset of its results was authored). Multiple authors could be provided. |
| A.1.8 Document metadata | Document metadata |
| A.1 Report header data elements (eHN) | A.1 Report header data elements (eHN) |
| A.1.3 Health insurance and payment information | Health insurance information is not always required, however, in some jurisdictions, the insurance number is also used as the patient identifier. It is necessary not just for identification but also forms access to funding for care. |
| A.1.4 Information recipient | Intended recipient or recipients of the report, e.g., GP, another specialist. Multiple recipients could be provided. |
| A.1.6 Legal authenticator | The person taking responsibility for the medical content of the document. |
| A.1.2 Patient/subject related contact information | Patient/subject related contact information |
| A.1.1 Identification of the patient/subject | Identification of the patient/subject |
| A.1.7 Result validator | Result validator |
| A.7 Comparison study | Comparison study |
| A.2 Order information | Note: an imaging report could respond to multiple orders. |
| A.5.3 Adverse reaction | Adverse reactions manifested during imaging investigation. |
| A.5.5 Conclusion | A concise and clinically contextualised summary including interpretation/impression of the diagnostic report |
| A.5 Examination Report | Examination Report |
| A.5.1 Imaging procedure description | Note: this part records the technical details of the performed procedures and may include information about protocol, imaging phases, imaging device, anatomical location, performer, place, date and time of performance. |
| A.5.2 Medication | Medication section includes information about medication administered during the medical imaging examination (contrast, sedation, stress agents), etc. |
| A.5.6 Recommendation | Recommendation |
| A.5.4 Results | Note: The results summarise the findings and observations by the health professional following the imaging study. Note: this part includes textual as well as structured results or findings of the imaging investigation. |
| A.6 Key images associated with this report | Key images associated with this report |
| A.8 Presented form | Presented form |
| A.3 Order Reason | Note: an imaging report could respond to multiple reasons. |
| A.4 Specimen information | Note: a specimen (not attached to a body) can be used for diagnostic, forensic and medical research purposes. |
The following FHIR ConceptMaps describe the way the logical model has been mapped onto the FHIR profiles defined in this specification.
| Title | Description |
| A.1.1 Identification of the patient/subject mapping | This concept map defines the mapping between the A.1.1 Identification of the patient/subject section and the FHIR profiles. |
| A.1.2 Patient/subject related contact information mapping | This concept map defines the mapping between the A.1.2 Patient/subject related contact information section and the FHIR profiles. |
| A.1.3 Health Insurance and Payment Information mapping | This concept map defines the mapping between the A.1.3. Health Insurance and Payment Information section and the FHIR profiles. |
| A.1.4 Information Recipient | This concept map defines the mapping between the A.1.4 Information Recipient section and the FHIR profiles. |
| A.1.5 Information Author | This concept map defines the mapping between the A.1.5 Information Author section and the FHIR profiles. |
| A.1.6 Legal Authenticator | This concept map defines the mapping between the A.1.6 Legal Authenticator section and the FHIR profiles. |
| A.1.7 ResultValidator | This concept map defines the mapping between the A.1.7 Result Validator section and the FHIR profiles. |
| A.1.8 Document Metadata | This concept map defines the mapping between the A.1.8 Document Metadata section and the FHIR profiles. |
| A.2 Order Information to FHIR profiles | This concept map defines the mapping between the A.2 Order Information section and the FHIR profiles. |
| A.3 Order Reason to FHIR profiles | This concept map defines the mapping between the A.3 Order Reason section and the FHIR profiles. |
| A.4 Specimen | This concept map defines the mapping between the A.3 Order Reason section and the FHIR profiles. The Specimen relates to a different type of information stored in a PACS: digital pathology. This has to analysed in more detail before inclusion in the IG. One of the things that might be impacted is the structure of the report. |
| A.5.1 Imaging Procedure Description | This concept map defines the mapping between the A.3 Order Reason section and the FHIR profiles. The rationale related to representing procedure phases can be found in Design consideration: Procedure Phases. |
| A.5.2 Imaging Procedure Medication | This concept map defines the mapping between the eHN A.5.2-ImagingProcedureMedication section and the FHIR profiles.
The medication information is mapped on the FHIR |
| A.5.3 Adverse reaction | This concept map defines the mapping between the eHN A.5.3 Adverse reaction section and the FHIR profiles.
In FHIR, adverse reactions are modelled using the |
| A.5.4 Results | This concept map defines the mapping between the eHN A.5.4 Results section and the FHIR profiles.
In FHIR, these are mapped on |
| A.5.5 Conclusion | This concept map defines the mapping between the eHN A.5.5 Conclusion section and the FHIR profiles.
The coded conclusions and narrative are mapped directly on |
| A.5.6 Recommendation | This concept map defines the mapping between the eHN A.5.6 Recommendation section and the FHIR profiles.
The eHN Guideline requires narrative recommendations only. It also states that in the future it is expected to also contain structured and coded recommendations. In order to support a transition towards a structured and coded format, the mapping is defined to the FHIR |
| A.6 Key images associated with this report | This concept map defines the mapping between the eHN A.6 Key images associated with this report section and the FHIR profiles. A key choice in modelling this dataset is the resources to use, the model is based on the design consideration on key image representation, both the DocumentReference and ImagingSelection resources are used to represent the key images. |
| A.7 Comparison Study | This concept map defines the mapping between the eHN A.7 Comparison Study section and the FHIR profiles. |
| A.8 Comparison Study | This concept map defines the mapping between the eHN A.8 Comparison Study section and the FHIR profiles. |
| B Comparison Study | This concept map defines the mapping between the eHN B Comparison Study section and the FHIR profiles. |