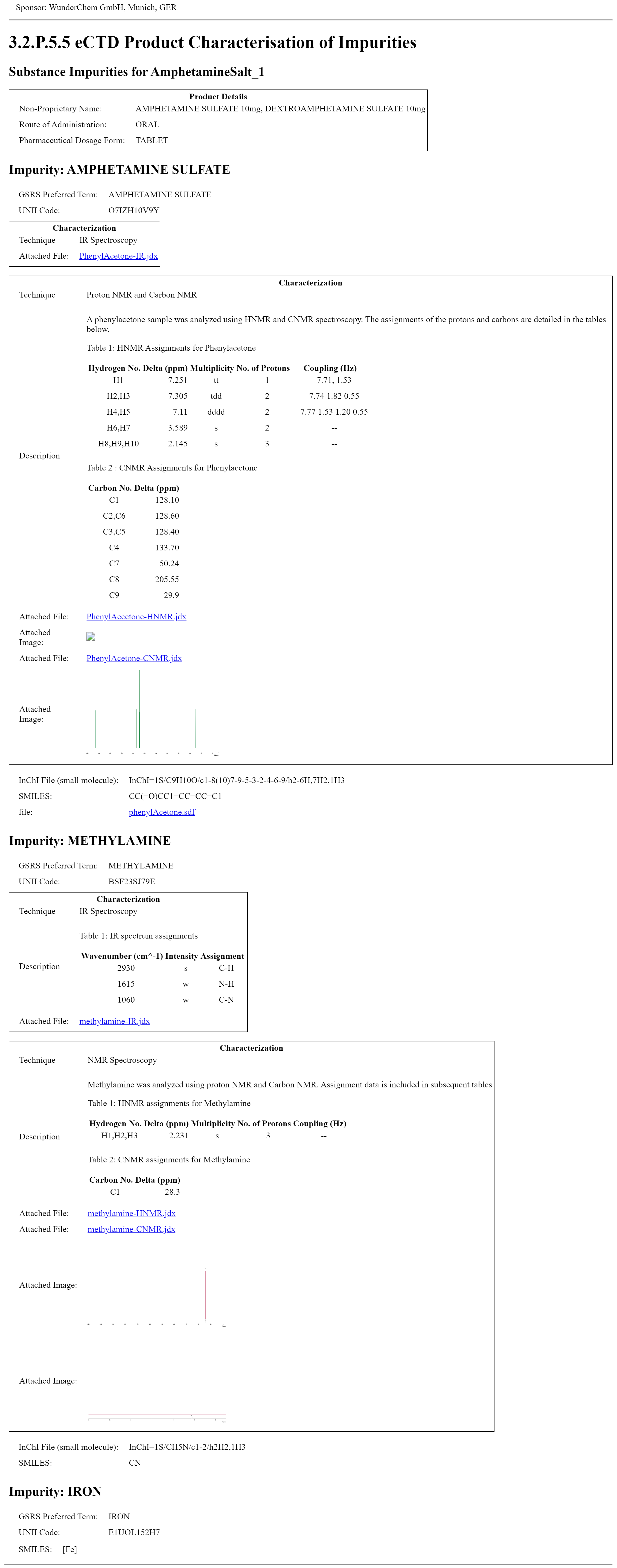
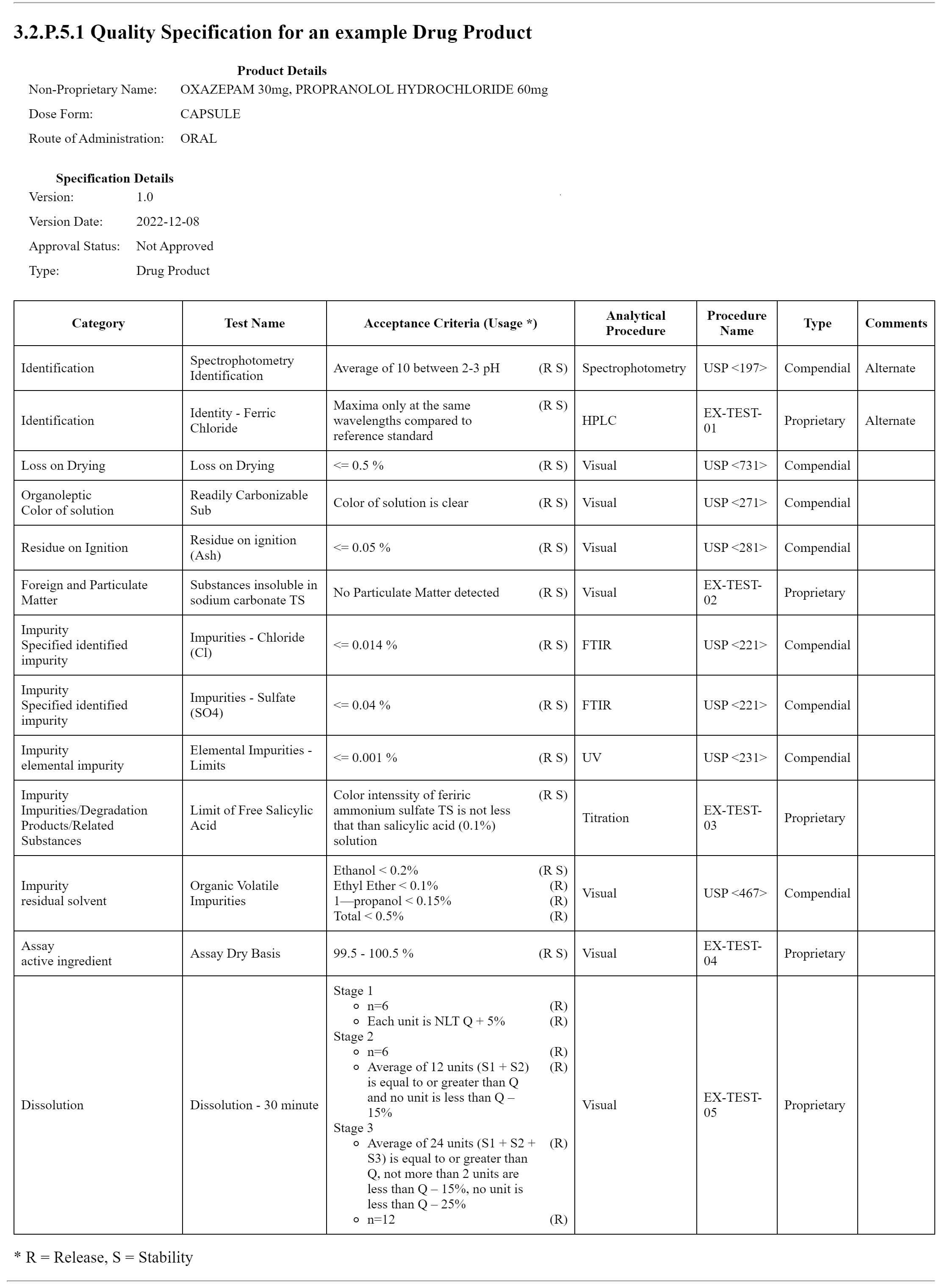
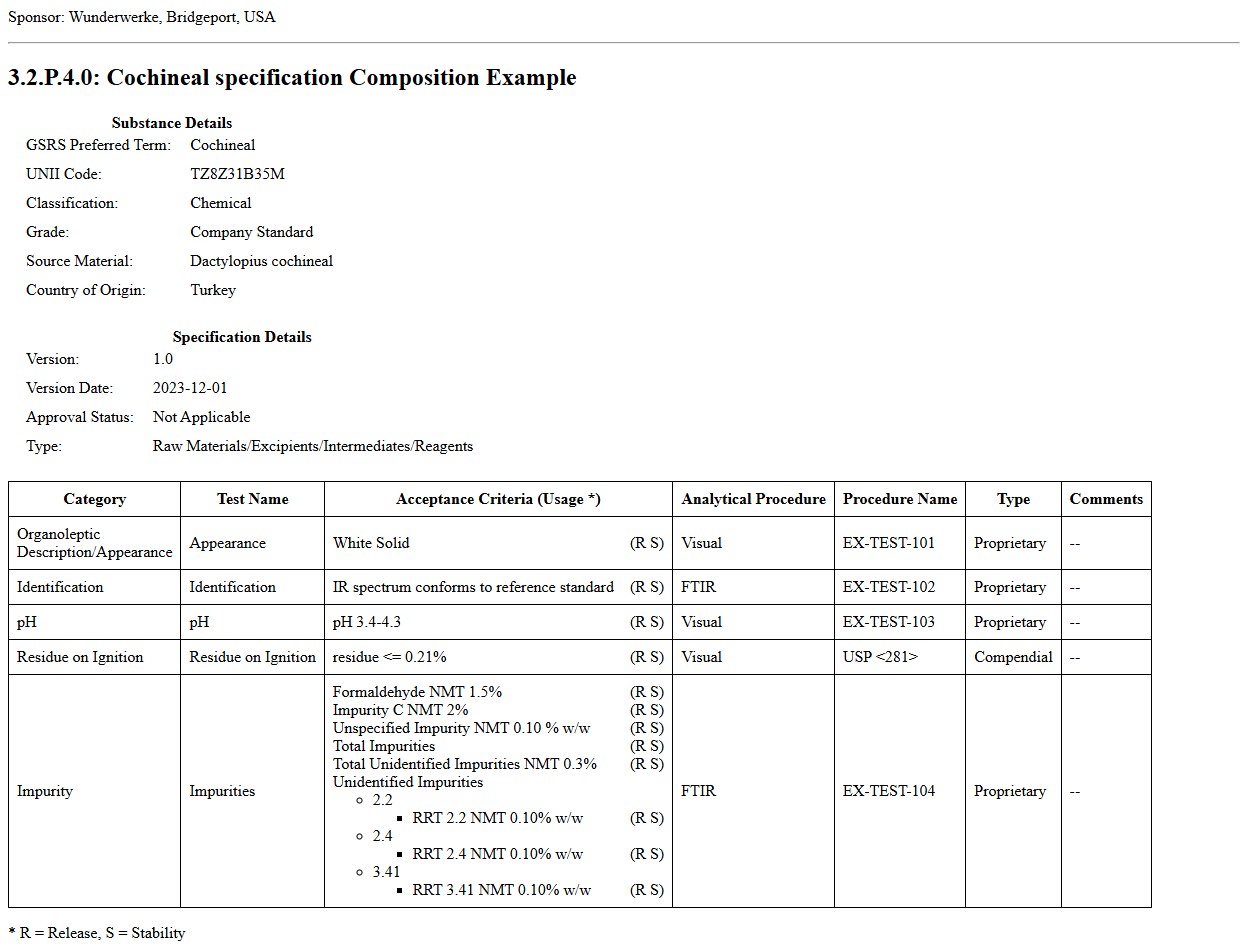
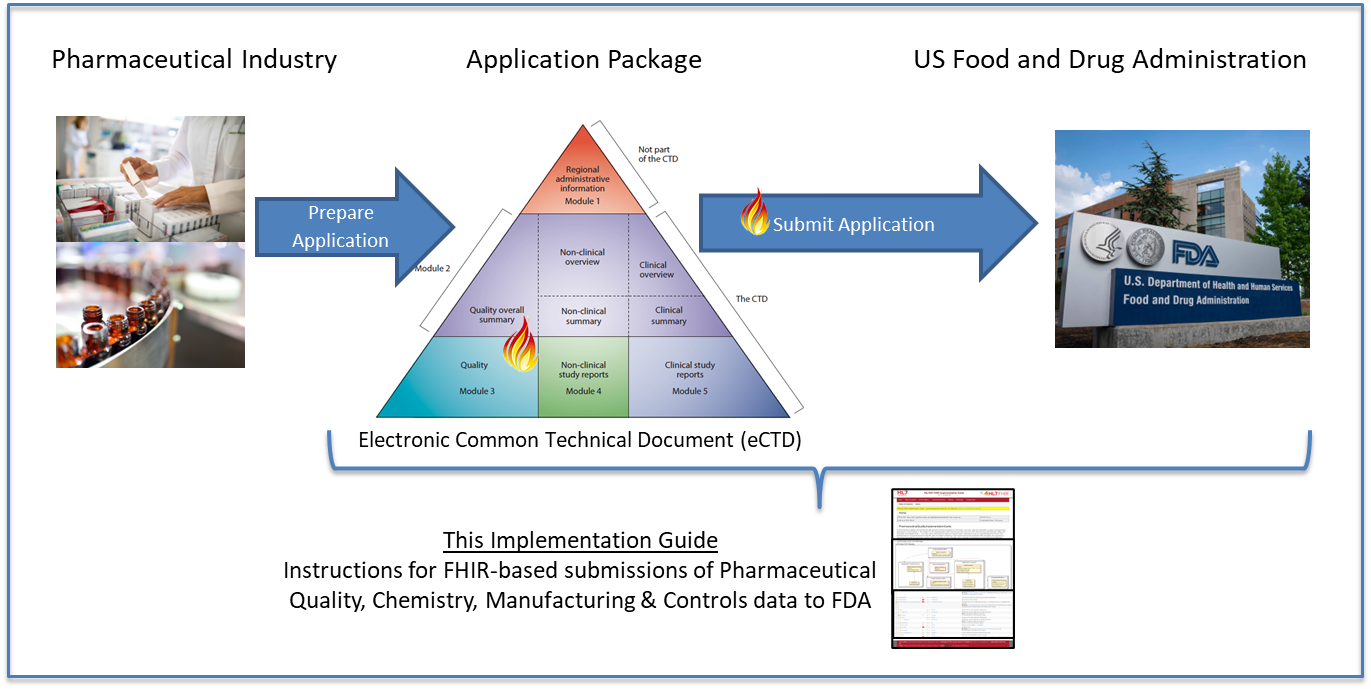


This fragment is available on index.html
This publication includes IP covered under the following statements.
| Type | Reference | Content | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| web | unitsofmeasure.org |
Include these codes as defined in
http://unitsofmeasure.org
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | unitsofmeasure.org |
Include these codes as defined in
http://unitsofmeasure.org
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | github.com | Pharmaceutical Quality - Chemistry, Manufacturing and Controls (PQ-CMC) Submissions to FDA, published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 2.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/FHIR-us-pq-cmc-fda/ and changes regularly. See the Directory of published versions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | en.wikipedia.org | Both Bundle.link and Bundle.entry.link are defined to support providing additional context when Bundles are used (e.g. HATEOAS ). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | www.ich.org | Drug product application content, including PQ/CMC, is harmonized internationally by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ( ICH ). The content standard for drug product applications is the ICH Common Technical Document (CTD) guideline which also has an ICH Topic ID of M4 (Multidisciplinary Guideline 4). The PQ/CMC content within the M4 Topic is identified as the Quality content and the section of the M4 guideline specific to PQ-CMC content is M4Q. When revision numbers are included, the resulting identifier is M4Q(R1), M4Q(R2), etc. The M4 guideline associates a “module” framework with numbers so that Module 2.3 is the Quality Overall Summary (one of several summaries in Module 2) and Module 3 is the Quality Data section (see the ICH CTD page ). The M4Q CTD guideline describes the content of both CTD Modules 2.3 and 3. Note that ICH terms typically use a British English spelling. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | ich.org | Drug product application content, including PQ/CMC, is harmonized internationally by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ( ICH ). The content standard for drug product applications is the ICH Common Technical Document (CTD) guideline which also has an ICH Topic ID of M4 (Multidisciplinary Guideline 4). The PQ/CMC content within the M4 Topic is identified as the Quality content and the section of the M4 guideline specific to PQ-CMC content is M4Q. When revision numbers are included, the resulting identifier is M4Q(R1), M4Q(R2), etc. The M4 guideline associates a “module” framework with numbers so that Module 2.3 is the Quality Overall Summary (one of several summaries in Module 2) and Module 3 is the Quality Data section (see the ICH CTD page ). The M4Q CTD guideline describes the content of both CTD Modules 2.3 and 3. Note that ICH terms typically use a British English spelling. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | www.ich.org | ICH created an associated guideline to harmonize the electronic exchange of CTD content (e.g., sections or subsections in pdf, xml, etc.) which is called the electronic Common Technical Document ( eCTD ) and is identified as the ICH M8 Topic (Multidisciplinary Guideline 8). Within the ICH eCTD (M8) guideline, the format and technology for exchange is defined. The eCTD guideline provides the regulatory application exchange framework in which FHIR-based CTD content “document bundles” will be exchanged between industry and the FDA. NOTE : Details about how to configure an eCTD submission to use FHIR-based PQ/CMC document bundles are outside of the scope of this IG. The eCTD is designed to reflect CTD concepts and the two acronyms are often used interchangeably. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | ich.org |
"The information, material and photographic content provided on this website are protected by copyright and may, with the exception of the ICH logo, be used, reproduced, incorporated into other works, adapted, modified, translated or distributed under a public license provided that ICH's copyright in the information and material is acknowledged at all times. In case of any adaption, modification or translation of the information, material or photographic content, reasonable steps must be taken to clearly label, demarcate or otherwise identify that changes were made to or based on the original information or material. Any impression that the adaption, modification or translation of the original information or material is endorsed or sponsored by the ICH must be avoided."For more information, see
https://ich.org/page/legal-mentions
Show Usage
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | ich.org | https://ich.org/page/legal-mentions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | www.iso.org |
ISO maintains the copyright on the country codes, and controls its use carefully. For further details see the ISO 3166 web page: https://www.iso.org/iso-3166-country-codes.html
Show Usage
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| web | ucum.org |
The UCUM codes, UCUM table (regardless of format), and UCUM Specification are copyright 1999-2009, Regenstrief Institute, Inc. and the Unified Codes for Units of Measures (UCUM) Organization. All rights reserved. https://ucum.org/trac/wiki/TermsOfUse
Show Usage
|
32P102layers.png 
|
32S10Lev.png 
|
APIspec.png 
|
BatchFormula.png 
|
ProdImpurities.png 
|
ProdSpec.png 
|
excipient.png 
|
figure1.png 
|
figure2.png 
|
figure3.png 
|
image1.png 
|
image2.png 
|
image3.png 
|
materials.png 
|
substanceCharacter.png 
|
tree-filter.png 
|