Consolidated CDA Release 2.1 StructureDefinition Publication, published by Health Level Seven. This is not an authorized publication; it is the continuous build for version 2.1). This version is based on the current content of https://github.com/HL7/CDA-ccda-2.1-sd/ and changes regularly. See the Directory of published versions
The C-CDA Companion Guide provides additional guidance aimed at increasing consistency in the way content in structured C-CDA documents is represented and used across all implementations. The goal is to raise awareness about why and how to use the section templates defined in C-CDA, and in other standards that supplement and complement C-CDA, to meet rising expectations and emerging regulations focused on expanding interoperability.
This chapter provides general guidance relevant when representing clinical information as structured C-CDA documents. It explains the purpose of structured sections and supplies background on how the section templates in C-CDA were originally developed. It summarizes the set of sections defined by Consolidate CDA and explains how each type of document template uses different section templates to represent —in human readable form– the information commonly contained in each specific type of clinical note. It introduces the section templates which require machine processable “discrete data” to accompany the human readable narrative. Finally, it summarizes several section templates that are new for Consolidated C-CDA or defined in implementation guides published elsewhere in the C-CDA implementer community and provides guidance on their use in C-CDA documents.
The idea of improving the communication of information contained in clinical notes has been around for decades. Long before C-CDA, a documentation methodology called “SOAP (Subjective, Objective, Assessment, and Plan) notes” was invented. The birth of the problem-oriented medical record (POMR) and SOAP note marked an epoch in the history of health care. Dr. Lawrence Weed, developer of the SOAP note and professor of medicine and pharmacology at Yale University, challenged conventional medical documentation and advocated for a scientific structure to frame clinical reasoning in the 1950s.^81 Today, the SOAP note is the most common method of documentation used by providers to input notes into patients’ medical records. They allow providers to record and share information in a universal, systematic and easy to read format.^82 Ineffective communication contributes to the top causes of sentinel events and continues to be an unremitting area for refinement.^83
After the development of the HL7 Clinical Document Architecture R2 standard in 2005, an alliance of healthcare vendors, providers and associations pooled resources in a rapid-development initiative. In a span of three years, the Health Story Project produced eight Health Level Seven (HL7) data standards for the flow of information using common types of healthcare documents. The alliance examined thousands of common clinical notes generated by a variety of medical transcription solutions and identified what structured sections were needed to represent the SOAP notes commonly generated by current-day clinicians. The “Health Story Guides,” as they were originally named, defined the initial set of section templates based on this analysis. Every section template includes a section.text element designed to hold the human readable narrative of that section of the structured document.
While no longer active today, the HIMSS Health Story Project provided education to the health IT community on tools and resources to aid in the creation of comprehensive electronic records that tell a patient’s complete health story. Some especially educational concepts from this initiative are summarized in the following chapters.
This roundtable presentation explained the twelve common clinical document types that are defined in Consolidated CDA (C-CDA) and described how each can be leveraged for information exchange in different care settings and for different encounter types. When should each C-CDA clinical document type be leveraged? What sections are used in C-CDA clinical documents and how do they differ from a Continuity of Care Document (CCD)? The Roundtable showed how to apply the SOAP framework to reveal the storytelling power of C-CDA by thinking of the section templates in terms of the subjective, objective, assessment, and plan information each contains. In some cases, the information in one section may be classified in multiple SOAP categories. The categorization is an approximation designed to improve understanding of the full collection of C-CDA section templates.
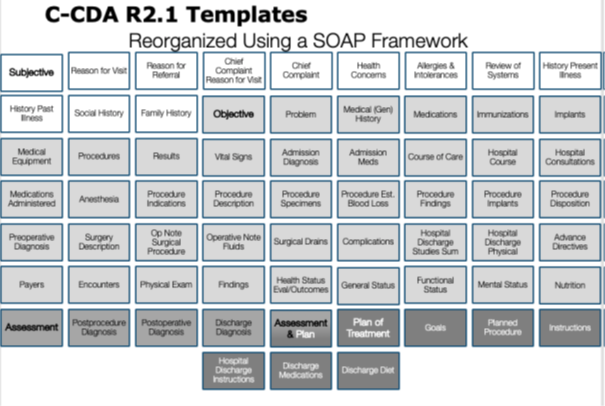 |
Figure 5: Visualizing C-CDA section templates by applying the SOAP framework
This presentation explained how documentation-based exchange via Consolidated Clinical Document Architecture (C-CDA), when implemented correctly, has the power to capture and share a more comprehensive electronic record that can be used to improve care.
By 2011, when C-CDA R1.0 was first published, a larger industry effort brought together CDA templates for clinical SOAP notes that had been defined by several organizations including Integrating the Healthcare Enterprise (IHE), HITSP, and HL7. The harmonized work included not only definitions for section templates needed to structure common clinical note types, but also included entry templates that defined how to represent the human readable section information using machine processable “clinical statements”. Clinical statements were templated using the syntax and data structures supported by the HL7 CDA R2 standard which derived its data types and modeling from an early version of the HL7 Reference Information Model (RIM). The HL7 RIM is the cornerstone of all HL7 Version 3 standards. It is a shared model between all domains and, as such, forms a common basis from which all domains can create information exchange artifacts and messages.^84
Today in C-CDA and in the C-CDA Supplemental Implementation Guides, the C-CDA standards development community has defined and published a wide array of section templates to represent clinical information in the context of structured documents. It is important to note that the purpose of each section template is dependent on the context of its intended use within a larger document structure. While some templates have been defined generically and are suitable for reuse in multiple structured documents, others due to the nature of their definitions may not be appropriate for re-use across other documents. For this reason, is it important for the context of the overarching document to be considered when determining if it is appropriate to include a particular type of section template in a particular type of document template.
C-CDA Content Creators SHOULD use section templates that are appropriate within the context of a document based on the defined purpose of the section template. [BP-054] |
The following guidance elements are not specific to any one C-CDA template but rather are overarching guidance elements that apply to an entire C-CDA document.
The author role is key to understanding the provenance of the information.
The roles populated in the header of the document apply to each section of content as well, unless explicitly indicated otherwise. If the author information for a section is not explicitly declared, then the author of the information in that section can be assumed to be the author contained in the document header. This is behavior within a CDA document is called context conduction.
This assumption extends to entries contained in the section as well. If the author information for an entry is not explicitly declared, then the author of the information in that entry can be assumed to be the author contained in the encompassing section.
While it is generally preferred that provenance be conveyed either at the document level, or at the entry level, it is possible for provenance to be conveyed at the section level when the default context conduction does not apply.
When representing a clinical note using structured sections, each section of information receives its context from the document’s header. If author information for a section is not explicitly declared, then the author of that section of information can be assumed to be the same as the author information contained in the document header.
The recordTarget, author, and informant roles populated in the header of the document apply to each section of content as well, unless explicitly indicated otherwise. The author role is key to understanding the provenance of the information in the document. If the author information for a section is not explicitly declared, then the author of that section of information can be assumed to be the same as the author information contained in the header.
This assumption extends to entries contained in the section as well, unless the author information is explicitly declared at the entry level. While it is generally preferred that provenance be conveyed at the entry level, provenance information included at the document or section level conducts to the entry when provenance at the entry level is not stated explicitly.
As explained in Chapter 2.3.1 Declaring Template Conformance, template conformance may be declared at any level of a C-CDA document—header, section, entry, or within an entry at a sub-structural level.
A template declaration in a C-CDA section asserts the constraints applicable for that section of XML. The template declaration tells validators and Content Consumers what to expect in terms of the information that, may, should, or shall be populated within this section of the document.
C- CDA templates are identified with a templateId. The templateId is a two-part identifier that consists of a root and an extension. The root identifies the named template and the extension identifies the version of that template. Initially C-CDA templates did not include versions. The templateId/@extension attribute was not used. Many of those original template versions are still used in C-CDA R2.1.
Chapter 3.1.2 of the Consolidated CDA Implementation Guide discusses the use of templateIds and what needs to be included in a C-CDA document:
Over time, many implementations have added template declarations without regard for the overhead they place on validation services or the confusion they may create for Content Consumers. The volume of templateId declarations adds to the size and complexity of C-CDA documents. This is beginning to have a negative impact for the C-CDA implementer community and has resulted in the realization that templateId declarations need regular maintenance and pruning over time.
C-CDA R2.1 Content Creators SHOULD NOT declare conformance to irrelevant templates. [BP-066] |
C-CDA R2.1 Content Creators SHALL NOT include duplicate templateId declarations. [CONF-067] |
A duplicate templateId declaration is a template declaration for the same structural part of a C-CDA document (i.e., document, section, entry, entry-part) with identical @root and @extension information. The order of the @root and @extension attributes does not matter when determining duplication.
To avoid confusion and minimize inclusion of unnecessary information in C-CDA documents, implementers should avoid including duplicate or irrelevant templateId declarations.
It is important to note that including the 2.1 templateid and the 1.1 template id is no duplication and is valid to describe the content as conformant to both the 1.1 and 2.1 versions of C-CDA.
As standards evolve an implementer community may decide to deprecate a version of a template or a collection of templates published in a version of an implementation guide. It should be anticipated that implementer communities may determine that certain version of a template should not be used going forward.
C-CDA Content Creators and C-CDA Content Consumers SHOULD NOT attribute semantic meaning to templateId declarations in C-CDA documents. [BP-057] |
C-CDA Content Creators SHOULD support template deprecation requiring discontinued use of all versions of a templateId or a specific version of a templateId. [BP-058] |
Example 16: Declaring template conformance at the section level.
<!-- Allergies and Intolerances Section-->
<component>
<section>
<!-- Conformant to C-CDA R2.1 Allergies and Intolerances Section-->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1" extension="2015-08- 01"/>
<!-- Conformant to C-CDA R1.1 Allergies and Intolerances Section -->
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<id root="0937FF9A-00CE-11E6-B4C5-0050568B000B"/>
<code code="48765-2" displayName="Allergies &or adverse reactions Doc"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Allergies</title>
<text>
</text>
</section>
</component>
Example 17: Declaring conformance to multiple templates.
<!—Substance or Device Allergy -->
<templateId root="2.16.840.1.113883.10.20.24.3.90" extension="2014-06- 09" />
<templateId root="2.16.840.1.113883.10.20.24.3.90"/>
<!— Allergy – Intolerance Observation -->
<templateId root="2.16.840.1.113883.10.20.22.4.7" extension="2014-06- 09" />
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
Example 18: (Wrong) Duplicate template declarations at the section level.
<!-- Allergies and Intolerances Section-->
<component>
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.6.1"/>
<templateId extension="2015-08- 01" root="2.16.840.1.113883.10.20.22.2.6.1" />
<id root="0937FF9A-00CE-11E6-B4C5-0050568B000B"/>
<code code="48765-2" displayName="Allergies &or adverse reactions Doc"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Allergies</title>
<text>
</text>
</section>
</component>
To support backwards compatibility, and in alignment with best practices documented in C-CDA 2.1 Volume 1 Section 3.1.2 Assertion of Compatibility, when utilizing templates defined in Appendix A of the Companion Guide that are newer versions of templates previously published in C-CDA 2.1, implementers should include both the templateId for the new version of the template, as well as the templateId for prior version of the template published in C-CDA 2.1.
Figure 6: Example of how to include templateIds for Companion Guide templates
<observation classCode="OBS" moodCode="EVN">
<!-- ** Problem Observation ** -->
<!-- Problem Observation (C-CDA 1.1) -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" />
<!-- Problem Observation (C-CDA 2.1) -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2015-08- 01" />
<!-- Problem Observation (C-CDA Companion Guide R3) -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" extension="2022- 06 - 01" />
<id root="ab1791b0-5c71-11db-b0de-0800200c9a66" />
...
</observation>
The CDA requirement for human readability guarantees that a receiver of a CDA document can algorithmically display the clinical content of the note on a standard Web browser.^85 This requirement impacts C-CDA in the following ways:
These principles and requirements have led to the current approach, where the material to be rendered is placed into the section.text field. In some cases, the data design of the entry templates required in the section heavily influence what clinical information can be expected to be present in the Narrative Block. In other cases, where entry templates are optional or not defined at all, the content in the Narrative Block may reflect information gathered in source systems as text and human crafted notes.
If the CDA Body is structured, the Content Creator includes the attested narrative content in the appropriate section.text field, regardless of whether information is also conveyed in CDA entries. An originator of a CDA document is not required to fully encode all narrative into CDA entries within the CDA body. Within specific implementations, trading partners may ascribe additional originator responsibilities to create various entries that meet certain conformance requirements or meet the conformance requirements described by defined templates.
^85 HL7 CDA. 1.2.3 Human Readability and Rendering CDA Documents.
Example 19: Sample Narrative Block in a Section
<section>
...
<text>
<table>
<colgroup>
<col width="25%"/>
<col width="25%"/>
<col width="25%"/>
<col width="25%"/>
</colgroup>
<thead>
<tr>
<th>Information Type</th>
<th>Date</th>
<th>Relevant Information</th>
<th>Documented By</th>
</tr>
</thead>
<tbody>
<tr ID="SocialHistory_1">
<td>Tobacco smoking status:</td>
<td><content>(04/12/2016)</content></td>
<td><content>Never smoked</content></td>
<td><content>M.Smith</content> <content>(04/12/2016)</content></td>
</tr>
</tbody>
</table>
</text>
...
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.78"/>
<templateId root="2.16.840.1.113883.10.20.22.4.78"
extension="2014-06- 09"/>
<id extension="64020-Z9301" root="1.2.840.114350.1.13.6289.1.7.1.1040.1"/>
<code code="72166-2" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Tobacco smoking status"/>
<text><reference value="#SocialHistory_1"></reference></text>
<statusCode code="completed"/>
<effectiveTime value="20160412"/>
<value xsi:type="CD" code="266919005" codeSystem="2.16.840.1.113883.6.96"
displayName="Never smoked tobacco" />
<author>
<time value="20160412"/>
<assignedAuthor>
<id extension="10.1" root="1.2.840.114350.1.1"/>
</assignedAuthor>
</author>
</observation>
</entry>
...
</section>
CDA permits additional information conveyed in the document that is there primarily for machine processing that is not authenticated and need not be rendered. However, the requirement that all attested content be present in the section.text field, suggests best practice is for all clinical content to be rendered through section.text.
C-CDA Content Creators SHOULD include all clinical content conveyed by a document in the section.text field. [BP-059] |
Reference: Representation of Discrete Data
The content model of the CDA Narrative Block schema is specially hand crafted to meet the requirements outlined above. Chapter 4.3.5 of the HL7 CDA standard provides a detailed explanation of the section Narrative Block and its representation to support ubiquitous rendering.^86 The table below provides information on some of the formatting tags commonly used to organize and render the human readable information in the Narrative Block.
| Tag Description | Description |
|---|---|
| <content> | The CDA |
| <br> | The CDA element is used to indicate a hard line break. It differs from the CDA element has no content. Receivers are required to interpret this element when rendering so as to represent a line break. |
| <list> | A CDA
|
| <table> | The CDA <table> is similar to the HTML table. The table markup is for presentation purposes only and, unlike a database table, does not possess meaningful field names. |
| <linkHtml> | The CDA |
| styleCode attribute | The styleCode attribute is used within the CDA Narrative Block to give the instance author the ability to suggest rendering characteristics of the nested character data (e.g. Bold, Underline, Italics). Receivers are not required to render documents using the style hints provided and can present stylized text in accordance with their local style conventions. Reference: HL7 CDA Chapter 4.3.5.11 for information on additional stylecodes. |
Table 27: Tags for formatting Narrative Text
Experience sharing documents has proven that different users expect, even demand, different views. Patients and providers have different needs; specialists and general practice providers have different needs. The HL7 Relevant and Pertinent Survey identified the need to improve rendering capabilities for the information contained in CDA documents.
CDA documents support a technique for using multiple XML stylesheet processing instructions and a controlled vocabulary for the @styleCode attribute has been established by IHE.^87 For more information on the use of styleCodes, reference the IHE Multiple Content Views (MCV) Profile - Published 2014- 08 -28. The profile includes examples to show how these styleCodes can be used to improve rendering options.
To promote a more consistent user experience for the viewing of the human readable content, implementers are encouraged to use the set of @styleCode values established by the MCV Profile. These @styleCode values establish a common way to tag text as a code, a date, a dateTime, an alert, and many other generally useful concepts.
^87 For more information on the use of styleCodes, reference the IHE Multiple Content Views (MCV) Profile - Published 2014-08-28. The profile includes examples to show how these styleCodes can be used to improve rendering options.
These styleCode values can be used to facilitate multiple clinical content rendering features. Systems that create CDA documents can use values from this table to improve the processing and rendering options for the information. Systems that render CDA document content with these styleCodes shall not omit or hide or otherwise obstructed from view information that uses these styleCodes unless they are using a specific rendering view that calls for such behavior.
Temporal information is often included as relevant and pertinent information to be exchanged. However, implementers need to be diligent when representing date/time information to make sure irrelevant or inaccurate levels of precision are not introduced when representing this type of information.
Reference: 5.1.10 Detailed Date/Time Guidance
Sharing irrelevant data, or omitting relevant data, can have an undesirable impact on clinician satisfaction and/or patient care. Developers of software to create, render, or incorporate C-CDA documents are encouraged to review recommendations in the HL7 CDA® R2 IG: Clinical Summary Relevant and Pertinent Data, Release 1 (PI ID: 1183) that underwent ballot during the HL7 January 2017 ballot cycle.
Again, it is important to emphasize the reusability and flexibility of templates so that implementations support the ability to tailor the content in CDA sections specific to the patient’s care, provider, or setting needs. Within C-CDA, nearly all templates allow additional content and are described as open templates.
While section templates define the content to be included in that structural component of the document, additional content may augment each document as needed for a particular circumstance, so long as the content is relevant to the defined purpose of the section.
For example, the Social History Section is defined as, “ This section contains social history data that influence a patient’s physical, psychological, or emotional health (e.g., smoking status, pregnancy). Demographic data, such as marital status, race, ethnicity, and religious affiliation, is captured in the header.”^88
Therefore, information about the person’s level of education, housing instability, food insecurity or transportation access challenges could be included in this section, provided the document did not contain another section defined to include this information more appropriately.
Existing templates defined in C-CDA can be used to represent additional data elements defined in the Vocabulary section of the Interoperability Standards Advisory (ISA). When using available templates to represent a data element defined in the ISA, it is important to represent the concept with coded values from the associated value set included for that data element in the ISA. Additionally, as use cases warrant it, implementers can consider including CDA templates from outside the C-CDA standard itself, such as those from the Supplemental Implementation Guides which define new template versions and templates for additional use cases, These Supplemental Implementation Guides are published alongside the main C-CDA specification: https://www.hl7.org/implement/standards/product_brief.cfm?product_id=492.
Caution: Implementers should be aware that recipients may not understand templates included at an unexpected location within a document.
^88 HL7 C-CDA R2.1 Chapter 2.66 Social History Section.
In order to communicate that business rules have been applied to constrain the amount of information represented in the section of a document C-CDA Content Creators should explicitly clarify the time range of the included information in the human readable text for the section.
For example, the Vital Signs Section is defined as, ”The Vital Signs Section contains relevant vital signs for the context and use case of the document type, such as blood pressure, heart rate, respiratory rate, height, weight, body mass index, head circumference, pulse oximetry, temperature, and body surface area. The section should include notable vital signs such as the most recent, maximum and/or minimum, baseline, or relevant trends. Vital signs are represented in the same way as other results but are aggregated into their own section to follow clinical conventions.”^89
A C-CDA Content Creator may have a business rule that limits the amount of vital sign information included in a Patient Summary to only the most recent vital signs for the patient in the requested period of time. This may be determined based on the effectiveTime/high for the time interval of the request. Business rules that further constrain the standard purpose of the defined section template should be explicitly documented in the section content.
Reference : 5.1.8 Specifying Time Intervals for Sections with Limits on the Included Discrete Data
The Section Time Range Observation is a newly defined template to describe the business rule used to limit the information contained in the section. It is an optional entry and may be used in any section.
useful when a query for a C-CDA document may request a large amount of data–potentially years—and the system that creates the document supplied in a response, limits the data they return to a specific range of time. This template enables the system creating the document to state the business logic used to constrain the amount of data provided in a section.
The Section Time Range template is used to communicate the ‘business logic’ used to limit the information contained in the section to a specific range of time. For example, if a CCD document is requested for the last 5 years and the Content Creator system has business rules that creates documents on demand but only returns one- year of past laboratory results, the Section Time Range template can be used to indicate the laboratory section only contains 12 months of data from the requested effectiveTime/low or at most 12 months of data prior to the effectiveTime/high in the request. The business logic used to limit the data is stated in the value element of the Section Time Range template using a datatype of IVL_TS. The template does not include an effectiveTime element.
^89 HL7 C-CDA R2.1. Chapter 2.70 Vital Signs Section.
Example 20: Example of Section Time Range Observation
<section>
...
<title>Procedures</title>
<text>
<content ID="Proc_STR">Procedures performed between 08/15/2012 and 08/15/2015.</content>
...
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.201" extension="2016-06- 01"/>
<code code="82607-3" codeSystem="2.16.840.1.113883.6.1"
displayName="Section Date and Time Range"/>
<text>
<reference value="#Proc_STR"/>
</text>
<statusCode code="completed"/>
<value xsi:type="IVL_TS">
<low value="20120815"/>
<high value="20150815"/>
</value>
</observation>
</entry>
</section>
Reference: Appendix A.2
As explained in the Health Story Roundtable presentation titled “The Storytelling Power of C-CDA”, understanding the purpose of the C-CDA section templates is not facilitated by considering them in alphabetical order.^90 The C- CDA Implementation Guide presents them in alphabetical order to speed access for readers. Considering the C- CDA section templates using the SOAP framework makes it easier to see how these sections can be used in a structured document to express the content of a clinical SOAP note.
The application of the SOAP framework does not produce a perfect classification result. Some sections don’t fit well into the SOAP framework. They have been classified as “other types of sections”. Some sections are defined to contain “heterogeneous” information, meaning the section’s content spans the boundaries of the SOAP framework. For example, the Assessment and Plan Section contains both assessment (A) and plan (P) information. Section structures that contain “homogeneous” information, all of the same type with the same purpose, improves information processing.
^90 https://www.himss.org/sites/hde/files/HSP%20March%202019%20Roundtable%20Q%26A.pdf
C-CDA Content Creators SHOULD use section templates containing “homogeneous” information with regards to not mixing subjective, objective, assessment, and plan types of information together in a single section. [BP-060] |
| Section Name LOINC OID |
Purpose Description | |
|---|---|---|
| Subjective Section 61150-9 2.16.840.1.113883.10.20.21.2.2 |
This section describes in a narrative format the patient’s current condition and/or interval changes as reported by the patient or by the patient’s guardian or another informant. | |
| Reason for Visit 29299-5 2.16.840.1.113883.10.20.22.2.12 |
This section records the patient’s reason for the patients’ visit (as documented by the provider). Local policy determines whether Reason for Visit and Chief Complaint are in separate or combined sections. | |
| Reason for Referral 42349-1 1.3.6.1.4.1.19376.1.5.3.1.3.1:2014-06-09 |
This section describes the clinical reason why a provider is sending a patient to another provider for care. The reason for referral may become the reason for visit documented by the receiving provider. | |
| Chief Complaint 10154-3 2.16.840.1.113883.10.20.22.2.13 |
This section records the patient’s chief complaint (the patient’s own description). | |
| Chief Complain and Reason for Visit 46239-0 2.16.840.1.113883.10.20.22.2.13 |
This section records the patient’s chief complaint (the patient’s own description) and/or the reason for the patient’s visit (the provider’s description of the reason for visit). Local policy determines whether the information is divided into two sections or recorded in one section serving both purposes. Reference: Chapter 4.3 Sections Defined in C-CDA |
|
| Health Concerns Section 75310-3 2.16.840.1.113883.10.20.22.2.58:2015-08-01 |
This section contains data describing an interest or worry about a health state or process that could possibly require attention, intervention, or management. A Health Concern is a health-related matter that is of interest, importance or worry to someone, who may be the patient, patient’s family or patient’s health care provider. Health concerns are derived from a variety of sources within IEHR. Health concerns can be medical, surgical, nursing, allied health or patient-reported concerns. “Transportation difficulties” for someone who doesn’t drive and has trouble getting to appointments, or “Underinsured” for someone who doesn’t have sufficient insurance to properly cover their medical needs such as medications. Problem Concerns are a subset of Health Concerns that have risen to the level of importance that they typically would be described in the Problems Section. |
|
| Allergies and Intolerances Section 48765-2 2.16.840.1.113883.10.20.22.2.6.1:2015-08-01 |
This section lists and describes any medication allergies, adverse reactions, idiosyncratic reactions, anaphylaxis/anaphylactoid reactions to food items, and metabolic variations or adverse reactions/allergies to other substances (such as latex, iodine, tape adhesives). At a minimum, it should list currently active and any relevant historical allergies and adverse reactions. | |
| Review of Systems Section 10187-3 1.3.6.1.4.1.19376.1.5.3.1.3.18 |
This section contains a relevant collection of symptoms and functions systematically gathered by a clinician. It includes symptoms the patient is currently experiencing, some of which were not elicited during the history of present illness, as well as a potentially large number of pertinent negatives, for example, symptoms that the patient denied experiencing. | |
| History of Present Illness 10164-2 1.3.6.1.4.1.19376.1.5.3.1.3.4 |
This section describes the history related to the reason for the encounter. It contains the historical details leading up to and pertaining to the patient’s current complaint or reason for seeking medical care. | |
| Past Medical History 11348-0 2.16.840.1.113883.10.20.22.2.20:2015-08-01 |
This section contains a record of the patient’s past complaints, problems, and diagnoses. It contains data from the patient’s past, up to the patient’s current complaint or reason for seeking medical care. | |
| Social History Section 29762-2 2.16.840.1.113883.10.20.22.2.17:2015-08-01 |
This section contains social history data that influence a patient’s physical, psychological or emotional health (e.g., smoking status, pregnancy, work). Demographic data, such as marital status, race, ethnicity, and religious affiliation, is captured in the header. | |
| Family History Section 10157-6 2.16.840.1.113883.10.20.22.2.15:2015-08-01 |
This section contains data defining the patient’s genetic relatives in terms of possible or relevant health risk factors that have a potential impact on the patient’s healthcare risk profile. |
Table 28: Subjective Information
| Sections defined in C-CDA R2.1 LOINC OID |
Purpose Description |
|---|---|
| Objective Section 61149-1 2.16.840.1.113883.10.20.21.2.1 |
This section contains data about the patient gathered through tests, measures, or observations that produce a quantified or categorized result. It includes important and relevant positive and negative test results, physical findings, review of systems, and other measurements and observations. |
| Problems Section 11450-4 2.16.840.1.113883.10.20.22.2.5.1:2015-08-01 |
This section lists and describes all relevant clinical problems at the time the document is generated. At a minimum, all pertinent current and historical problems should be listed. Overall health status may be represented in this section. |
| Medical (General) History Section 11329-0 2.16.840.1.113883.10.20.22.2.39 |
This section describes all aspects of the medical history of the patient even if not pertinent to the current procedure, and may include chief complaint, past medical history, social history, family history, surgical or procedure history, medication history, and other history information. The history may be limited to information pertinent to the current procedure or may be more comprehensive. The history may be reported as a collection of random clinical statements or it may be reported categorically. Categorical report formats may be divided into multiple subsections including Past Medical History, Social History. |
| Medications Section 10160-0 2.16.840.1.113883.10.20.22.2.1.1:2014-06-09 |
This section contains a patient’s current medications and pertinent medication history. At a minimum, the currently active medications are listed. An entire medication history is an option. The section can describe a patient’s prescription and dispense history and information about intended drug monitoring. |
| Immunizations Section 11369-6 2.16.840.1.113883.10.20.22.2.2.1:2015-08-01 |
This section defines a patient’s current immunization status and pertinent immunization history. The primary use case for the Immunization Section is to enable communication of a patient’s immunization status. The section should include current immunization status and may contain the entire immunization history that is relevant to the period of time being summarized. |
| Medical Equipment Section 46264-8 2.16.840.1.113883.10.20.22.2.23:2014-06-09 |
This section defines a patient’s implanted and external health and medical devices and equipment. This section lists any pertinent durable medical equipment (DME) used to help maintain the patient’s health status. All equipment relevant to the diagnosis, care, or treatment of a patient should be included. Devices applied to, or placed in, the patient are represented with the Procedure Activity Procedure template. Equipment supplied to the patient (e.g., pumps, inhalers, wheelchairs) is represented by the Non-Medicinal Supply Activity template. These devices may be grouped together within a Medical Equipment Organizer. |
| Procedures Section 47519-4 2.16.840.1.113883.10.20.22.2.7.1:2014-06-09 |
This section describes all interventional, surgical, diagnostic, or therapeutic procedures or treatments pertinent to the patient historically at the time the document is generated. The section should include notable procedures. |
| Results Section 30954-2 2.16.840.1.113883.10.20.22.2.3.1:2015-08-01 |
This section contains the results of observations generated by laboratories, imaging and other procedures. The scope includes observations of hematology, chemistry, serology, virology, toxicology, microbiology, plain x-ray, ultrasound, CT, MRI, angiography, echocardiography, nuclear medicine, pathology, and procedure observations. This section often includes notable results such as abnormal values or relevant trends. |
| Vital Signs Section 8716-3 2.16.840.1.113883.10.20.22.2.4.1:2015-08-01 |
This section contains relevant vital signs for the context and use case of the document type, such as blood pressure, heart rate, respiratory rate, height, weight, body mass index, head circumference, pulse oximetry, temperature, and body surface area. The section should include notable vital signs such as the most recent, maximum and/or minimum, baseline, or relevant trends. Vital signs are represented in the same way as other results. However, they are represented in their own section to follow clinical conventions. |
| Course of Care Section 8648-8 2.16.840.1.113883.10.20.22.2.64 |
This section describes what happened during the course of an encounter. |
| General Status Section 10210-3 2.16.840.1.113883.10.20.2.5 |
This section describes general observations and readily observable attributes of the patient, including affect and demeanor, apparent age compared to actual age, gender, ethnicity, nutritional status based on appearance, body build and habitus (e.g., muscular, cachectic, obese), developmental or other deformities, gait and mobility, personal hygiene, evidence of distress, and voice quality and speech. |
| Functional Status Section 47420-5 2.16.840.1.113883.10.20.22.2.14:2014-06-09 |
This section contains observations and assessments of a patient’s physical abilities. A patient’s functional status may include information regarding the patient’s ability to perform Activities of Daily Living (ADLs) in areas such as Mobility (e.g., ambulation), Self-Care (e.g., bathing, dressing, feeding, grooming) or Instrumental Activities of Daily Living (IADLs) (e.g., shopping, using a telephone, balancing a check book). Problems that impact function (e.g., dyspnea, dysphagia) can be contained in the section. |
| Mental Status Section 10190-7 2.16.840.1.113883.10.20.22.2.56:2015-08-01 |
This section contains observations and evaluations related to a patient’s psychological and mental competency and deficits including, but not limited to any of the following types of information: • Appearance (e.g., unusual grooming, clothing or body modifications) • Attitude (e.g., cooperative, guarded, hostile) • Behavior/psychomotor (e.g., abnormal movements, eye contact, tics) • Mood and affect (e.g., anxious, angry, euphoric) • Speech and Language (e.g., pressured speech, perseveration) • Thought process (e.g., logic, coherence) • Thought content (e.g., delusions, phobias) • Perception (e.g., voices, hallucinations) • Cognition (e.g., memory, alertness/consciousness, attention, orientation) – which were included in Cognitive Status Observation in earlier publications of C-CDA.• Insight and judgment (e.g., understanding of condition, decision making) |
| Nutrition Section 61144-2 2.16.840.1.113883.10.20.22.2.57 |
This section represents diet and nutrition information including special diet requirements and restrictions (e.g., texture modified diet, liquids only, enteral feeding). It also represents the overall nutritional status of the patient and nutrition assessment findings. |
| Specific to Inpatient Encounter Notes | |
| Admission Diagnosis Section 46241-6 2.16.840.1.113883.10.20.22.2.43:2015-08-01 |
This section contains a narrative description of the problems or diagnoses identified by the clinician at the time of the patient’s admission. This section may contain a coded entry which represents the admitting diagnoses. |
| Admission Medications Section 42346-7 2.16.840.1.113883.10.20.22.2.44:2015-08-01 |
This section contains the medications taken by the patient prior to and at the time of admission to the facility. |
| Hospital Course Section 8648-8 1.3.6.1.4.1.19376.1.5.3.1.3.5 |
This section describes the sequence of events from admission to discharge in a hospital facility. |
| Hospital Consultations Section 18841-7 2.16.840.1.113883.10.20.22.2.42 |
This section records consultations that occurred during the admission. |
| Hospital Discharge Studies Summary Section 11493-4 2.16.840.1.113883.10.20.22.2.16 |
This section records the results of observations generated by laboratories, imaging procedures, and other procedures. The scope includes hematology, chemistry, serology, virology, toxicology, microbiology, plain x-ray, ultrasound, CT, MRI, angiography, echocardiography, nuclear medicine, pathology, and procedure observations. This section often includes notable results such as abnormal values or relevant trends and could record all results for the period of time being documented. |
| Hospital Discharge Physical Section 10184-0 1.3.6.1.4.1.19376.1.5.3.1.3.2 |
This section records a narrative description of the patient’s physical findings generated by the discharge physician at the time of discharge. |
| Discharge Medications Section 10183-2 2.16.840.1.113883.10.20.22.2.11.1:2015-08-01 |
This section contains the medications the patient is intended to take or stop after discharge. Current, active medications must be listed. The section may also include a patient’s prescription history and indicate the source of the medication list. |
| Specific to Procedure and Operative Notes | |
| Medications Administered Section 29549-3 2.16.840.1.113883.10.20.22.2.38:2014-06-09 |
This section usually resides inside a Procedure Note describing a procedure. This section defines medications and fluids administered during the procedure, its related encounter, or other procedure related activity excluding anesthetic medications. |
| Anesthesia Section 59774-0 2.16.840.1.113883.10.20.22.2.25:2014-06-09 |
This section records the type of anesthesia (e.g., general or local) and may state the actual agent used. This may be a subsection of the Procedure Description Section. The full details of anesthesia are usually found in a separate Anesthesia Note. |
| Procedure Indications Section 59768-2 2.16.840.1.113883.10.20.22.2.29:2014-06-09 |
This section contains the reason(s) for the procedure or surgery. This section may include the pre-procedure diagnoses as well as symptoms contributing to the reason for the procedure. |
| Medical Equipment Section 46264-8 urn:hl7ii:2.16.840.1.113883.10.20.22.2.23:2014-06-09 |
This section contains devices that have been placed in a patient. This section is also relevant for recording information about non-implanted medical equipment and non-medicinal supplied equipment (e.g. wheelchair, hearing aid, walker). Reference: 5.2.8 Medical Equipment |
| Complications Section 55109-3 2.16.840.1.113883.10.20.22.2.37:2015-08-01 |
This section contains problems that occurred during or around the time of a procedure. The complications may be known risks or unanticipated problems. |
Table 29: Objective Information
| Sections defined in C-CDA R2.1 LOINC OID |
Purpose Description |
|---|---|
| Assessment Section 51848-0 2.16.840.1.113883.10.20.22.2.8 |
This section (also referred to as “impression” or “diagnoses” outside of the context of CDA) represents the clinician’s conclusions and working assumptions that will guide treatment of the patient. The assessment may be a list of specific disease entities or a narrative block. |
| Assessment and Plan Section 51847-2 2.16.840.1.113883.10.20.22.2.9:2014-06-09 |
This section represents the clinician’s conclusions and working assumptions that will guide treatment of the patient. The Assessment and Plan Section may be combined or separated to meet local policy requirements. Best practice is to separate these distinct types of information by using the Assessment Section: templateId 2.16.840.1.113883.10.20.22.2.8 and the Plan of Treatment Section: templateId 2.16.840.1.113883.10.20.22.2.10:2014-06-09 Reference: Chapter 4.3 Sections Defined in C-CDA |
| Specific to Inpatient Encounter Notes: | |
| Discharge Diagnosis Section 11535-2 2.16.840.1.113883.10.20.22.2.24:2015-08-01 |
This section represents problems or diagnoses present at the time of discharge which occurred during the hospitalization. This section includes an optional entry to record patient diagnoses specific to this visit. Problems that need ongoing tracking should also be included in the Problem Section. |
| Specific to Procedure and Operative Notes: | |
| Postprocedure Diagnosis Section 59769-0 2.16.840.1.113883.10.20.22.2.36:2015-08-01 |
This section records the diagnosis or diagnoses discovered or confirmed during the procedure. Often it is the same as the preprocedure diagnosis or indication. |
| Postoperative Diagnosis Section 10219-4 2.16.840.1.113883.10.20.22.2.35 |
This section records the diagnosis or diagnoses discovered or confirmed during the surgery. Often it is the same as the preoperative diagnosis. |
Table 30: Assessment Information
| Sections defined in C-CDA R2.1 LOINC OID |
Purpose Description |
|---|---|
| Goals Section 61146-7 2.16.840.1.113883.10.20.22.2.60 |
This section represents patient Goals. A goal is a defined outcome or condition to be achieved in the process of patient care. Goals include patient-defined over-arching goals and health concern-specific or intervention-specific goals to achieve desired outcomes. |
| Advance Directives Section 42348-3 2.16.840.1.113883.10.20.22.2.21.1:2015-08-01 |
This section contains data defining the patient’s advance directives and any reference to supporting documentation, including living wills, healthcare proxies, and CPR and resuscitation status. If the referenced documents are available, they can be included in the exchange package. |
| Assessment and Plan Section 51847-2 2.16.840.1.113883.10.20.22.2.9:2014-06-09 |
This section represents the clinician’s conclusions and working assumptions that will guide treatment of the patient. The Assessment and Plan Section may be combined or separated to meet local policy requirements. Best practice is to separate these distinct types of information by using the Assessment Section: templateId 2.16.840.1.113883.10.20.22.2.8 and the Plan of Treatment Section: templateId 2.16.840.1.113883.10.20.22.2.10:2014-06-09 Reference: 4.3 Sections Defined in C-CDA |
| Plan of Treatment Section 18776-5 2.16.840.1.113883.10.20.22.2.10:2014-06-09 |
This section, formerly known as “Plan of Care”, contains data that define pending orders, interventions, encounters, services, and procedures for the patient. It is limited to prospective, unfulfilled, or incomplete orders and requests only. All active, incomplete, or pending orders, appointments, referrals, procedures, services, or any other pending event of clinical significance to the current care of the patient should be listed. |
| Instructions Sections 69730-0 2.16.840.1.113883.10.20.22.4.20:2014-06-09 |
This section can be used in several ways, such as to record patient instructions within a Medication Activity or to record fill instructions within a supply order. |
| Specific to Procedure and Operative Notes: | |
| Planned Procedures Section 59772-4 2.16.840.1.113883.10.20.22.2.30:2014-06-09 |
This section contains the procedure(s) that a clinician planned based on the preoperative assessment. |
| Specific to Procedure and Operative Notes: | |
| Hospital Discharge Instructions Section 8653-8 2.16.840.1.113883.10.20.22.2.41 |
This section records instructions at discharge. |
Table 31: Plan/Planning Information
| Sections defined in C-CDA R2.1 LOINC OID |
Purpose Description |
|---|---|
| Encounters Section 46240-8 2.16.840.1.113883.10.20.22.2.22.1:2015-08-01 2.16.840.1.113883.10.20.22.2.18:2015-08-01 |
This section is relevant in nearly all documents. It lists and describes any healthcare encounters pertinent to the patient’s current health status or historical health history. An encounter is an interaction, regardless of the setting, between a patient and a practitioner who is vested with primary responsibility for diagnosing, evaluating, or treating the patient’s condition. It may include visits, appointments, or non-face-to-face interactions. An encounter also may be a contact between a patient and a practitioner who has primary responsibility (exercising independent judgment) for assessing and treating the patient at a given contact. This section may include a single encounter in an Encounter Summary. It contains relevant encounters for the time period being summarized in a Patient Summary Document. |
| Payers Section 48768-6 2.16.840.1.113883.10.20.22.2.18:2015-08-01 |
This section contains data on the patient’s payers, “third party” insurance, self-pay, other payer or guarantor, or some combination of payers, and is used to define which entity is the responsible fiduciary for the financial aspects of a patient’s care. Each unique instance of a payer and all the pertinent data needed to contact, bill to, and collect from that payer should be included. Authorization information that can be used to define pertinent referral, authorization tracking number, procedure, therapy, intervention, device, or similar authorizations for the patient or provider, or both should be included. At a minimum, the patient’s pertinent current payment sources should be listed. |
Table 32: Other Information
Several section templates have been developed specifically to supplement C-CDA. They are defined within the
context of C-CDA Supplemental Guides that have been balloted and reconciled separately from C-CDA, and are
published alongside the main C-CDA Specification
https://www.hl7.org/implement/standards/product_brief.cfm?product_id=492. These guides are developed with
the intention of eventually replacing earlier version of the templates in C-CDA or eventually being added to be set
of templates considered a part of the C-CDA set of templates.
At the time of this publication, the following Supplemental Implementation Guides are published alongside the C- CDA specification:
The following table defines new section templates defined within the context of this Companion Guide.
| Section Name LOINC OID |
Purpose Description |
|---|---|
| Care Teams Section 85847-2 Entries optional: 2.16.840.1.113883.10.20.22.2.500:2022-06-01 |
The Care Team Section is used to share historical and current Care Team information. The Care Team Section may be included in any type of C-CDA structured document that is an open template. An individual can have more than one Care Team. A Care Team can exist over time such as a longitudinal care team which includes historical members that may be active or inactive on the care team as needed. Or a Care Team, such as a rehabilitation team, may exist to address a person’s needs associated with a particular care event, or a team can be based on addressing a specific condition. The Care Team Organizer entry template used in the C-CDA Care Teams Section is meant to support the foundation of effective communication, interaction channels and maintenance of current clinical context awareness for the patient, caregivers and care providers to promote care coordination. |
| Notes Section 34109-9 Entries required: 2.16.840.1.113883.10.20.22.2.65:2016-11-01 |
This section allows for inclusion of clinical documentation which does not fit precisely within any other C-CDA section. Multiple Notes sections may be included in a document provided they each include different types of note content as indicated by a different section.code. The Notes Section is not used in place of a more specific a C-CDA section. For example, notes about procedure should be placed within the Procedures Section, not a Notes Section. When a Notes Section is present, Note Activity entries contain structured information about the note information allowing it to be more machine processable. |
Table 33: New and Additional C-CDA Sections
The table below summaries other section templates available by reference from C-CDA Supplemental IGs and CDA R2 IGs developed by other stakeholders in the C-CDA implementer community.
NOTE: The HL7 CDA Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers Set 1, Release 1 – US Realm specification indicates which document types SHALL include the more tightly constrained or additional templates required for exchanging information with payers using C-CDA documents.^92
The table also summarizes any tighter expectations for requiring other C-CDA sections to be included in the structured documents.^93 Implementation guides developed within the Payer C-CDA implementer community tighten the constrains on four existing sections and defines three new sections.
Implementers with the Payer and broader HIT C-CDA implementer community have started utilizing section templates defined by the Quality C-CDA implementer community to express and exchange information about care gaps that exist for individual patients. The templates used for this use case are re-used from the QRDA Cat I implementation guide.
| Section Name LOINC OID |
Purpose Description |
|---|---|
| Measure Section QDM ^94 55186-1 2.16.840.1.113883.10.20.24.2.3 |
This section template contains information about the measure or measures being reported. This section references the measure through reference to an externalDocument. The externalDocument/ids and version numbers are used to reference the measure. The measure section must contain a reference to at least one externalDocument id of all the measures being reported in the QRDA instance. NOTE: Only measure information is included in this section. The clinical data that support quality calculation could be included in other sections (e.g. problems, procedures, results, vital signs, etc.) |
| Functional Status Section (CDP1)^96 47420-5 2.16.840.1.113883.10.20.22.2.14:2014-06-09 |
The Functional Status Section contains observations and assessments of a patient’s physical abilities. A patient’s functional status may include information regarding the patient’s ability to perform Activities of Daily Living (ADLs) in areas such as Mobility (e.g., ambulation), Self-Care (e.g., bathing, dressing, feeding, grooming) or Instrumental Activities of Daily Living (IADLs) (e.g., shopping, using a telephone, balancing a check book). Problems that impact function (e.g., dyspnea, dysphagia) can be contained in the section. |
| Plan of Treatment Section (CDP1)^97 18776-5 2.16.840.1.113883.10.20.35.2.6 |
This section, formerly known as “Plan of Care”, contains data that define pending orders, interventions, encounters, services, and procedures for the patient. It is limited to prospective, unfulfilled, or incomplete orders and requests only. This Plan of Treatment Section (CDP1) requires a response for all entry templates. It requires explicitly recording when information is not available in the source system to populate a required entry template. |
| Social History Section (CDP1)^98 29762-2 2.16.840.1.113883.10.20.35.2.7 |
This section contains social history data that influences a patient’s physical, psychological or emotional health (e.g. smoking status, pregnancy). Demographic data, such as marital status, race, ethnicity, and religious affiliation, is captured in the header. This Social History Section (CDP1) requires a response for all entry templates. It requires explicitly recording when information is not available in the source system to populate a required entry template. |
| Additional Documentation Section (CDP1)^99 77599-9 2.16.840.1.113883.10.20.35.4.11 |
The Additional Documentation Section (CDP1) contains additional documentation captured by the provider related to administrative requirements or care provided/planned for the patient, that is not supported in any other section of the document. (example: statement of no financial relationship with a service supplier).100 |
| Externally Defined CDE Section (CDP1)^101 77598-1 2.16.840.1.113883.10.20.35.2.2 |
The Externally Defined CDE Section (CDP1) contains externally defined Clinical Data Elements (CDEs) that have been created through the interaction of the provider with externally defined templates (or questionnaires) that define name-value pairs and a reference to the externally defined information/content model.102 |
| Orders Placed Section (CDP1)^103 77597-3 2.16.840.1.113883.10.20.35.2.3 |
The Orders Placed Section (CDP1) contains active and completed (not planned) orders for observations, interventions, encounters, services, and procedures for the patient. The entries in this section represent the details of the orders and not the acts involved in the processing and fulfilment of the order. This section includes order information in order to validate that clinical activities performed by other providers and suppliers are authorized by the responsible provider.104 Implementers may want to consider utilizing the Section Time Range template and apply business rules to limit the amount of information that would be included in this section. |
Table 34: Sections defined in other Implantation Guides
^93 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm, Table 24. page 125 ^94 HL7 QRDA Cat 1, Measure Section QDM ^95 HL7 CDA Examples Search. Quality Care Compliance in C-CDA. http://hl7-c-cda-examples.herokuapp.com/sections/Quality ^96 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 132. ^97 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 141. ^98 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 147. ^99 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 161. ^100 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 129 ^101 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 131. ^102 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 131. ^103HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm.Page 137. ^104 HL7 CDA® Release 2 Implementation Guide: Additional CDA R2 Templates – Clinical Documents for Payers – Set 1, Release 1 – US Realm. Page 137.