Consolidated CDA Release 2.1 StructureDefinition Publication, published by Health Level Seven. This is not an authorized publication; it is the continuous build for version 2.1). This version is based on the current content of https://github.com/HL7/CDA-ccda-2.1-sd/ and changes regularly. See the Directory of published versions
| Official URL: http://hl7.org/cda/us/ccda/StructureDefinition/MedicationFreeTextSig | Version: 2.1 | |||
| Draft as of 2023-09-29 | Computable Name: MedicationFreeTextSig | |||
| Other Identifiers: id: urn:oid:2.16.840.1.113883.10.20.22.4.147 | ||||
The template is available to explicitly identify the free text Sig within each medication.
An example free text sig: Thyroxin 150 ug, take one tab by mouth every morning.
Usage:
Description of Profiles, Differentials, Snapshots and how the different presentations work.
This structure is derived from CDAR2.SubstanceAdministration
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | CDAR2.SubstanceAdministration | XML Namespace: urn:hl7-org:v3 Elements defined in Ancestors: @classCode, @moodCode, realmCode, typeId, templateId, id, code, @negationInd, text, statusCode, effectiveTime, priorityCode, repeatNumber, routeCode, approachSiteCode, doseQuantity, rateQuantity, maxDoseQuantity, administrationUnitCode, consumable, subject, specimen, performer, author, informant, participant, entryRelationship, reference, precondition | ||
  | 1..1 | cs | ||
  | 1..1 | cs | moodCode must match the parent substanceAdministration EVN or INT Binding: MoodCodeEvnInt (required) | |
  | 1..* | II | Slice: Unordered, Closed by value:root | |
  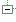 | 1..1 | II | ||
   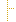 | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.10.20.22.4.147 | |
   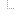 | 0..0 | |||
  | 1..1 | CD | ||
   | 1..1 | cs | Required Pattern: 76662-6 | |
  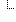 | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.6.1 | |
  | 1..1 | ED | ||
   | 1..1 | TEL | Reference into the section/text to a tag that only contains free text sig. | |
    | C | 0..1 | url | 81-32774: This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). |
  | 1..1 | InfrastructureRoot | ||
   | 1..1 | ManufacturedProduct | ||
    | 1..1 | LabeledDrug | ||
     | 1..1 | cs | Fixed Value: NA | |
 Documentation for this format Documentation for this format | ||||
| Path | Conformance | ValueSet |
| SubstanceAdministration.moodCode | required | MoodCodeEvnInt |
| Id | Grade | Path(s) | Details | Requirements |
| 81-32774 | error | SubstanceAdministration.text.reference.value | This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). : |
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 1..1 | CDAR2.SubstanceAdministration | XML Namespace: urn:hl7-org:v3 Elements defined in Ancestors: @classCode, @moodCode, realmCode, typeId, templateId, id, code, @negationInd, text, statusCode, effectiveTime, priorityCode, repeatNumber, routeCode, approachSiteCode, doseQuantity, rateQuantity, maxDoseQuantity, administrationUnitCode, consumable, subject, specimen, performer, author, informant, participant, entryRelationship, reference, precondition Base for all types and resources | |
  | 1..1 | cs | Binding: ActClass (required) Fixed Value: SBADM | |
  | 1..1 | cs | moodCode must match the parent substanceAdministration EVN or INT Binding: MoodCodeEvnInt (required) | |
  | 1..* | II | Slice: Unordered, Closed by value:root | |
   | 1..1 | II | ||
    | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.10.20.22.4.147 | |
  | 1..1 | CD | Binding: ActSubstanceAdministrationCode (extensible) | |
   | 1..1 | cs | Required Pattern: 76662-6 | |
   | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.6.1 | |
  | 1..1 | ED | ||
   | 1..1 | TEL | Reference into the section/text to a tag that only contains free text sig. | |
    | C | 0..1 | url | 81-32774: This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). |
  | 1..1 | InfrastructureRoot | ||
   | 1..1 | ManufacturedProduct | ||
    | 1..1 | LabeledDrug | ||
     | 1..1 | cs | Binding: NullFlavor (required) Fixed Value: NA | |
 Documentation for this format Documentation for this format | ||||
| Path | Conformance | ValueSet / Code |
| SubstanceAdministration.classCode | required | Fixed Value: SBADM |
| SubstanceAdministration.moodCode | required | MoodCodeEvnInt |
| SubstanceAdministration.code | extensible | ActSubstanceAdministrationCode |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.nullFlavor | required | Fixed Value: NA |
| Id | Grade | Path(s) | Details | Requirements |
| 81-32774 | error | SubstanceAdministration.text.reference.value | This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). : | |
| ele-1 | error | **ALL** elements | All FHIR elements must have a @value or children : hasValue() or (children().count() > id.count()) |
| Path | Conformance | ValueSet / Code |
| SubstanceAdministration.classCode | required | Fixed Value: SBADM |
| SubstanceAdministration.moodCode | required | MoodCodeEvnInt |
| SubstanceAdministration.templateId:primary.nullFlavor | required | NullFlavor |
| SubstanceAdministration.code | extensible | ActSubstanceAdministrationCode |
| SubstanceAdministration.code.nullFlavor | required | NullFlavor |
| SubstanceAdministration.text.nullFlavor | required | NullFlavor |
| SubstanceAdministration.text.compression | required | CompressionAlgorithm |
| SubstanceAdministration.text.integrityCheckAlgorithm | required | IntegrityCheckAlgorithm |
| SubstanceAdministration.text.reference.nullFlavor | required | NullFlavor |
| SubstanceAdministration.text.reference.use | required | AddressUse |
| SubstanceAdministration.statusCode | required | ActStatus |
| SubstanceAdministration.priorityCode | extensible | ActPriority |
| SubstanceAdministration.routeCode | extensible | RouteOfAdministration |
| SubstanceAdministration.approachSiteCode | extensible | ActSite |
| SubstanceAdministration.administrationUnitCode | extensible | AdministrableDrugForm |
| SubstanceAdministration.consumable.nullFlavor | required | NullFlavor |
| SubstanceAdministration.consumable.typeCode | required | Fixed Value: CSM |
| SubstanceAdministration.consumable.manufacturedProduct.classCode | required | Fixed Value: MANU |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.nullFlavor | required | Fixed Value: NA |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.classCode | required | Fixed Value: MMAT |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.determinerCode | required | Fixed Value: KIND |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.code | extensible | DrugEntity |
| Id | Grade | Path(s) | Details | Requirements |
| 81-32774 | error | SubstanceAdministration.text.reference.value | This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). : | |
| ele-1 | error | **ALL** elements | All FHIR elements must have a @value or children : hasValue() or (children().count() > id.count()) |
This structure is derived from CDAR2.SubstanceAdministration
Summary
Mandatory: 10 elements
Fixed Value: 1 element
Prohibited: 1 element
Slices
This structure defines the following Slices:
Differential View
This structure is derived from CDAR2.SubstanceAdministration
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | CDAR2.SubstanceAdministration | XML Namespace: urn:hl7-org:v3 Elements defined in Ancestors: @classCode, @moodCode, realmCode, typeId, templateId, id, code, @negationInd, text, statusCode, effectiveTime, priorityCode, repeatNumber, routeCode, approachSiteCode, doseQuantity, rateQuantity, maxDoseQuantity, administrationUnitCode, consumable, subject, specimen, performer, author, informant, participant, entryRelationship, reference, precondition | ||
  | 1..1 | cs | ||
  | 1..1 | cs | moodCode must match the parent substanceAdministration EVN or INT Binding: MoodCodeEvnInt (required) | |
  | 1..* | II | Slice: Unordered, Closed by value:root | |
   | 1..1 | II | ||
    | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.10.20.22.4.147 | |
    | 0..0 | |||
  | 1..1 | CD | ||
   | 1..1 | cs | Required Pattern: 76662-6 | |
   | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.6.1 | |
  | 1..1 | ED | ||
   | 1..1 | TEL | Reference into the section/text to a tag that only contains free text sig. | |
    | C | 0..1 | url | 81-32774: This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). |
  | 1..1 | InfrastructureRoot | ||
   | 1..1 | ManufacturedProduct | ||
    | 1..1 | LabeledDrug | ||
     | 1..1 | cs | Fixed Value: NA | |
 Documentation for this format Documentation for this format | ||||
| Path | Conformance | ValueSet |
| SubstanceAdministration.moodCode | required | MoodCodeEvnInt |
| Id | Grade | Path(s) | Details | Requirements |
| 81-32774 | error | SubstanceAdministration.text.reference.value | This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). : |
Key Elements View
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 1..1 | CDAR2.SubstanceAdministration | XML Namespace: urn:hl7-org:v3 Elements defined in Ancestors: @classCode, @moodCode, realmCode, typeId, templateId, id, code, @negationInd, text, statusCode, effectiveTime, priorityCode, repeatNumber, routeCode, approachSiteCode, doseQuantity, rateQuantity, maxDoseQuantity, administrationUnitCode, consumable, subject, specimen, performer, author, informant, participant, entryRelationship, reference, precondition Base for all types and resources | |
  | 1..1 | cs | Binding: ActClass (required) Fixed Value: SBADM | |
  | 1..1 | cs | moodCode must match the parent substanceAdministration EVN or INT Binding: MoodCodeEvnInt (required) | |
  | 1..* | II | Slice: Unordered, Closed by value:root | |
   | 1..1 | II | ||
    | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.10.20.22.4.147 | |
  | 1..1 | CD | Binding: ActSubstanceAdministrationCode (extensible) | |
   | 1..1 | cs | Required Pattern: 76662-6 | |
   | 1..1 | oid, uuid, ruid | Required Pattern: 2.16.840.1.113883.6.1 | |
  | 1..1 | ED | ||
   | 1..1 | TEL | Reference into the section/text to a tag that only contains free text sig. | |
    | C | 0..1 | url | 81-32774: This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). |
  | 1..1 | InfrastructureRoot | ||
   | 1..1 | ManufacturedProduct | ||
    | 1..1 | LabeledDrug | ||
     | 1..1 | cs | Binding: NullFlavor (required) Fixed Value: NA | |
 Documentation for this format Documentation for this format | ||||
| Path | Conformance | ValueSet / Code |
| SubstanceAdministration.classCode | required | Fixed Value: SBADM |
| SubstanceAdministration.moodCode | required | MoodCodeEvnInt |
| SubstanceAdministration.code | extensible | ActSubstanceAdministrationCode |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.nullFlavor | required | Fixed Value: NA |
| Id | Grade | Path(s) | Details | Requirements |
| 81-32774 | error | SubstanceAdministration.text.reference.value | This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). : | |
| ele-1 | error | **ALL** elements | All FHIR elements must have a @value or children : hasValue() or (children().count() > id.count()) |
Snapshot View
| Path | Conformance | ValueSet / Code |
| SubstanceAdministration.classCode | required | Fixed Value: SBADM |
| SubstanceAdministration.moodCode | required | MoodCodeEvnInt |
| SubstanceAdministration.templateId:primary.nullFlavor | required | NullFlavor |
| SubstanceAdministration.code | extensible | ActSubstanceAdministrationCode |
| SubstanceAdministration.code.nullFlavor | required | NullFlavor |
| SubstanceAdministration.text.nullFlavor | required | NullFlavor |
| SubstanceAdministration.text.compression | required | CompressionAlgorithm |
| SubstanceAdministration.text.integrityCheckAlgorithm | required | IntegrityCheckAlgorithm |
| SubstanceAdministration.text.reference.nullFlavor | required | NullFlavor |
| SubstanceAdministration.text.reference.use | required | AddressUse |
| SubstanceAdministration.statusCode | required | ActStatus |
| SubstanceAdministration.priorityCode | extensible | ActPriority |
| SubstanceAdministration.routeCode | extensible | RouteOfAdministration |
| SubstanceAdministration.approachSiteCode | extensible | ActSite |
| SubstanceAdministration.administrationUnitCode | extensible | AdministrableDrugForm |
| SubstanceAdministration.consumable.nullFlavor | required | NullFlavor |
| SubstanceAdministration.consumable.typeCode | required | Fixed Value: CSM |
| SubstanceAdministration.consumable.manufacturedProduct.classCode | required | Fixed Value: MANU |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.nullFlavor | required | Fixed Value: NA |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.classCode | required | Fixed Value: MMAT |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.determinerCode | required | Fixed Value: KIND |
| SubstanceAdministration.consumable.manufacturedProduct.manufacturedLabeledDrug.code | extensible | DrugEntity |
| Id | Grade | Path(s) | Details | Requirements |
| 81-32774 | error | SubstanceAdministration.text.reference.value | This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA Release 2, section 4.3.5.1) (CONF:81-32774). : | |
| ele-1 | error | **ALL** elements | All FHIR elements must have a @value or children : hasValue() or (children().count() > id.count()) |
This structure is derived from CDAR2.SubstanceAdministration
Summary
Mandatory: 10 elements
Fixed Value: 1 element
Prohibited: 1 element
Slices
This structure defines the following Slices: