Clinical Decision Support for Chronic Pain Management and Shared Decision-Making IG
0.1.0 - CI Build
Clinical Decision Support for Chronic Pain Management and Shared Decision-Making IG
0.1.0 - CI Build
Clinical Decision Support for Chronic Pain Management and Shared Decision-Making IG, published by CQF. This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/cds4cpm/ and changes regularly. See the Directory of published versions
| Acronym | Literal Translation | |——————|————————————————————————————-| | ACPA | American Chronic Pain Association | | AHRQ | Agency for Healthcare Research & Quality | | API | Application Programming Interface | | CAT | Computer Adaptive Testing | | CDC | Centers for Disease Control and Prevention | | CDS | Clinical Decision Support | | CNCP | Chronic Non-Cancer Pain | | CQL | Clinical Quality Language | | EHR | Electronic Health Record | | FHIR | Fast Healthcare Interoperability Resources | | HL7 | Health Level Seven | IRB | Institutional Review Board | | IT | Information Technology | | MEDD | Maximum Equivalent Daily Dose (see details on the Epic-specific calculation) | | MME/day | Morphine Milligram Equivalent per day (see CDC guideline) | | NLM VSAC | National Library of Medicine Value Set Authority Center | | MVP | Minimum Viable Product | | MyPAIN | My Pain Assessment and Information Needs | | PCCDS-LN | Patient Centered Clinical Decision Support Learning Network | | PDMP | Prescription Drug Monitoring Program | | PEG scale | Pain, Enjoyment, General Activity scale for pain | | PMD | Pain Management Dashboard | | PRO | Patient-Reported Outcome | | PROMIS | Patient-Reported Outcomes Measurement Information System | | SDD | System Design Document | | SDM | Shared Decision Making | | SDOH | Social Determinants of Health | | SHARE | Seek, Help, Assess, Reach, and Evaluate | | SMART | Substitutable Medical Applications, Reusable Technologies | | UCM | University of Chicago Medicine | | UI | User Interface | | VUMC | Vanderbilt University Medical Center |
Through its Accelerating Change and Transformation in Organizations and Networks III (ACTION III) initiative, the Agency for Healthcare Research & Quality (AHRQ) has elected to sponsor a project that investigates how shareable and interoperable clinical decision support (CDS) can be made actionable for patients and clinicians at scale. Embodied in prior work such as that of the Patient-Centered Clinical Decision Support Learning Network (PCCDS-LN) and CDS Connect, AHRQ has invested in patient-centered and shareable CDS, but these efforts face development and implementation challenges to wider adoption and use. The system and services for which a testing plan is outlined in this document seek to connect patients and providers, clinical best practices, novel delivery models, and evidence generators to demonstrate how to provide CDS in a standardized, publicly shareable form at scale for improving chronic pain management.
This system, to which this testing plan applies, includes two central services. First, a patient-facing CDS artifact termed My Pain Assessment and Information Needs (MyPAIN), which will handle the collection and transmission of chronic non-cancer pain (CNCP) assessment information (patient-reported outcomes; PROs), provide relevant educational content, and support a workflow designed to handle the collection and delivery of this information to support a shared decision-making (SDM) encounter for a patient and their provider. Partnering with MyPAIN is a provider-facing artifact termed PainManager. PainManager, a read-only resource, is based on the MITRE-developed Pain Management Summary/Dashboard (PMD) (Agency for Healthcare Research and Quality, n.d.-b), a CDS Connect (Agency for Healthcare Research and Quality, n.d.-a) artifact, and handles the presentation of information collected via MyPAIN along with some demographic, social determinants of health (SDOH), and prescription drug monitoring program (PDMP) data, and other clinically relevant information retrieved from an electronic health record (EHR). Together, these products form a system for the delivery of both patient- and provider-facing CDS to support SDM in CNCP patients at two sites, Vanderbilt University Medical Center (VUMC) and the University of Chicago Medicine (UCM).
This system is being implemented in primary care clinics as identified by our two site partners: VUMC and UCM. We will target patients with four common conditions in the pilot: chronic low back pain, osteoarthritis of the hip, osteoarthritis of the knee, and fibromyalgia.
Targeting these patients involves the creation of automated query and triggering mechanisms at each site that send MyPAIN invitations to patients who are eligible to receive the intervention. This document is intended to define and outline a testing plan for this system, which includes a patient-facing application, MyPAIN, and a provider-facing application, PainManager. The information provided by these applications is presented in MyPAIN as a patient-facing application and in PainManager as a dashboard application, both implemented as Substitutable Medical Applications, Reusable Technologies on Fast Healthcare Interoperability Resources (SMART on FHIR) applications. These SMART on FHIR applications are expected to be integrated with or interfaced to an EHR.
This testing plan includes a six-pronged approach to testing the MyPAIN and PainManager applications and their broader system. This approach will ensure that the components or containers function as a unit and together meet the necessary requirements of the end users.
The objective of the testing of the MyPAIN and PainManager applications is to ensure functionality of the system and all essential system requirements, including:
MyPAIN and PainManager are intended to work together to provide end-to-end support for SDM around chronic pain management. for the targeted conditions Key functions of this system include allowing patients to complete self-report measures and education materials, provide contextual information from the patient’s medical record accessed via that medical record’s patient portal, notify a provider when this information is completed, and present results of this information along with PDMP information wherever available in a dashboard to support an SDM encounter for chronic pain at the time of the patient visit. MyPAIN and PainManager are intended to be implemented together rather than standalone applications.
To summarize, within this system are the following:
The applications above are designed to comprise a standalone system that can be integrated into EHRs and into clinical workflows. MyPAIN is expected to be invoked via a patient portal (e.g., MyChart for Epic systems), and PainManager is expected to be accessed via an ambulatory EHR (e.g., Epic Ambulatory). The system will support interactions between patients and clinicians.
Use of MyPAIN is dependent on a patient accepting an invitation to access and use the application. This invitation is triggered according to a particular patient profile or “phenotype” within the EHR. This phenotype will include criteria that identify relevant diagnoses. Sites will need to determine their own best practices for configuring and implementing the phenotype and programming a triggering mechanism(s) for sending an invitation to patients. Strategies might include using an existing appointment reminder system infrastructure either within the EHR environment (UCM) or through an external appointment system (VUMC). Whatever the approach, these and future sites will determine how to identify the candidates who will receive a MyPAIN invitation.
In addition to MyPAIN and PainManager, the high-level system architecture consists of several supporting applications (called containers in the architectural approach taken for this project) that enable the functionality delivered by PainManager and MyPAIN. Exhibit 1 illustrates these main containers and their interactions:
Exhibit 1 - Architectural Overview for CDS4CPM
Exhibit 2 - MyPAIN Requirements
| Component | ID | Requirement | Function | Priority | Resource |
|---|---|---|---|---|---|
| MyPAIN | 1.1.1 | OAuth 2.0 specification is used to identify and authenticate the patient when accessing MyPAIN | Authentication and matching | Core | Patient matching by clinical identifier is conducted to validate the identity of the patient. NOTE: Assumes a SMART on FHIR solution. |
| MyPAIN | 1.2.1 | MyPAIN populated with patient data from EHR | Data Population | Core | Demographic information—Patient name, DOB, condition, visit doctor, date and time |
| MyPAIN | 1.3.1 | Patient completes self-report measure(s) | Data Collection | Core | Use PROMIS Pain Intensity SF-3a (75262-6 In the past 7 days - How intense was your pain at its worst?; 75261-8 In the past 7 days - How intense was your average pain?; 75260-0 What is your level of pain right now?) |
| MyPAIN | 1.3.2 | Patient completes self-report measure(s) | Data Collection | Core | Use PROMIS Pain Interference SF-4a (LOINC: 61758-9 In the past 7 days - How much did pain interfere with your day to day activities?; 61769-6 In the past 7 days - How much did pain interfere with work around the home?; 61773-8 In the past 7 days - How much did pain interfere with your ability to participate in social activities?; 61775-3 In the past 7 days - How much did pain interfere with your household chores?) |
| MyPAIN | 1.3.4 | Patient completes self-report measure(s) | Data Collection | Core | Patient records treatment hx (see MyPAIN wireframes) |
| MyPAIN | 1.3.5 | Patient completes self-report measure(s) | Data Collection | Core | Patient records activity goals and barriers |
| MyPAIN | 1.4.1 | Patient is presented with chronic pain education materials | Patient Education - Living with Chronic pain | Core | US Pain Foundation website: https://uspainfoundation.org/living-with-pain/—chronic pain and treatment options |
| MyPAIN | 1.6.1 | Patient is presented with SDM education materials | Patient Education - Chronic pain | Desired | American Chronic Pain Association ‘Car with four flat tires’ video: https://www.theacpa.org/acpa-car-with-four-flat-tires/—chronic pain management |
Exhibit 3 - PainManager Requirements
| Component | ID | Requirement | Function | Priority | Resource |
|---|---|---|---|---|---|
| PainManager | 2.1.1 | PainManager operates under the authentication provided by the EHR’s patient portal | PainManager is invoked within the authentication provided by the EHR patient portal | Core | NA |
| PainManager | 2.1.2 | Provider can verify whether PainManager displays the correct patient | PainManager displays patient identifiers within the application | Core | NA |
| PainManager | 2.3.1 | Provider can access a patient’s responses to the treatment history assessment | PainManager displays the results from the treatment history assessment | Core | Treatment history assessment |
| PainManager | 2.3.2 | Provider can access a patient’s responses to the PROMIS Pain Intensity Survey (SF-3a) | PainManager displays the results from the PROMIS Pain Intensity Survey (SF-3a) | Core | SF-3a |
| PainManager | 2.3.3 | Provider can access a patient’s responses to the PROMIS Pain Interference Survey (SF-4a) | PainManager displays the results from the PROMIS Pain Intensity Survey (SF-4a) | Core | SF-4a |
| PainManager | 2.3.4 | Provider can access a patient’s responses to two open-ended questions about activity goals and barriers | PainManager graphically displays the results from the previous three timepoints for each question in the SF-3a battery | Core | SF-3a |
| PainManager | 2.3.5 | Provider can access a patient’s answers to three previous timepoints for the PROMIS Pain Interference Survey (SF-4a) | PainManager graphically displays the results from the previous three timepoints for each question in the SF-4a battery | Core | SF-4a |
| PainManager | 2.3.6 | Provider can access a patient’s answers to three previous timepoints for the PROMIS Pain Intensity Survey (SF-3a) | PainManager displays the results from the project’s three open-ended questions | Core | TBD |
| PainManager | 2.3.7 | Provider can access a patient’s responses to one open-ended question about the location(s) of their pain | PainManager displays the results from the pain-location open-ended question | Core | TBD |
| PainManager | 2.4.1 | Provider can view education materials patient viewed (or not) | PainManager displays whether the ACPA “Car with Four Flat Tires” video (or not) | Core | Web log |
| PainManager | 2.4.2 | Provider can view education materials patient viewed (or not) | PainManager displays whether the patient viewed the US Pain Foundation website | Core | Web log |
| PainManager | 2.4.3 | Provider can access the education material a patient viewed (or not) | PainManager displays the ACPA “Four Flat Tires” video | Core | ACPA’s Four Flat Tires, US Pain Foundation |
| PainManager | 2.4.4 | Provider can access the education materials a patient viewed (or not) | PainManager displays the US Pain Foundation website | Core | US Pain Foundation website |
| PainManager | 2.5.1 | A provider receives an EHR notification (passive or semi-active) that a patient provided data via MyPAIN | PainManager (or the system) delivers a notification of the site’s choosing (e.g., BPA, SnapShot) | Important | NA |
| PainManager | 2.5.2 | A provider can open PainManager from the EHR notification (passive or semi-active) | The EHR (or the system) provides a means to open PainManager via the site’s choosing (e.g., BPA, SnapShot) | Important | NA |
| PainManager | 2.5.3 | A provider can access PainManager whether a patient entered data via MyPAIN | The EHR offers access to PainManager via a “persistent link” in the EHR (e.g., Links, BPA) | Important | NA |
| PainManager | 2.7.1 | Provider can access PDMP data | PainManager at UCM (ONLY) displays a link to access PDMP data | Important | Illinois PDMP via LogiCoy |
| PainManager | 2.7.2 | Provider can view PDMP data | PainManager at UCM (only) displays PDMP data | Important | Illinois PDMP via LogiCoy |
| PainManager | 2.12.2 | Providers can view flags, counts, tooltips, information icons, and URLs | Show “flags” (different icon), show counts (only if = 0), tooltips (need to be updated), information icons (information needs updating), and URLs (need updating) from Pain Management Summary | Desired | Pain Management Summary |
| PainManager | 2.13.1 | Physician reviews pertinent medical history - Risk Factors for Opioid-related Harms | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.13.2 | Physician reviews pertinent medical history - Conditions Associated with Chronic Pain | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.13.3 | Physician reviews pertinent medical history - Risk Factors | PainManager displays data from the relevant value set via NLM VSAC | Core | NA |
| PainManager | 2.14.1 | Physician reviews risk considerations - Most Recent MME | PainManager displays data from the relevant value set via NLM VSAC | Core | NA |
| PainManager | 2.14.1 | Physician notices red exclamation mark for opioid meds and rolls mouse over | PainManager displays data from the relevant value set via NLM VSAC | Important | NA |
| PainManager | 2.14.2 | Physician reviews risk considerations - Urine Drug Screens | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.14.3 | Physician reviews risk considerations - Benzodiazepine Medications | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.14.4 | Physician reviews risk considerations - Naloxone Medications | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.14.5 | Physician reviews risk considerations - Risk Screenings Relevant to Pain Management | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.13.1 | Physician reviews pertinent medical history - Risk Factors for Opioid-related Harms | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.16.1 | Physician can view relevant SDOH data for the patient | PainManager displays patient-specific SDOH data (if available) | Core | Epic |
| PainManager | 2.17.1 | Physician can view why PainManager is offered for the patient | PainManager displays this information at the top of the summary display | Epic | |
| PainManager | 2.18.1 | Physician reviews Current and Historical Pain Treatments - Current Opioid Medications | PainManager displays data from the relevant value set via NLM VSAC | Core | NA |
| PainManager | 2.18.2 | Physician reviews Current and Historical Pain Treatments - Opioid Medications in the past year | PainManager displays data from the relevant value set via NLM VSAC | Core | NA |
| PainManager | 2.18.3 | Physician reviews Current and Historical Pain Treatments - Non-Opioid Medications | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.18.4 | Physician reviews Current and Historical Pain Treatments - Non-Pharmacologic Treatments | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
| PainManager | 2.18.5 | Physician reviews Current and Historical Pain Treatments - Stool Softeners and Laxatives | PainManager displays data from the relevant value set via NLM VSAC | Core | NLM VSAC |
Exhibit 4 - Phenotype Requirements
| Component | ID | Requirement | Function | Priority | Resource |
|---|---|---|---|---|---|
| Phenotype | 3.1.1 | A custom phenotype (TBD) specification is used to identify the patient to access MyPAIN | Custom Phenotype + trigger | Core | Services in English only |
| Phenotype | 3.1.2 | A custom phenotype (TBD) specification is used to identify the patient to access MyPAIN | Custom Phenotype + trigger | Core | Four specific conditions |
| Phenotype | 3.1.4 | A custom phenotype specification for VUMC used to exclude patients for access to MyPAIN | Custom Phenotype + trigger | Core | Identify patients (VUMC only) on opioids with an MME >= 20 per day |
| Phenotype | 3.1.5 | A custom phenotype (TBD) specification is used to identify the patient to access MyPAIN | Custom Phenotype + trigger | Core | ICD-10 codes for relevant conditions |
| Phenotype | 3.1.6 | A custom phenotype (TBD) specification is used to exclude patients for access to MyPAIN | Custom Phenotype + trigger | Core | Identify patients (VUMC only) on opioids enrolled in INSPIRE trial |
| Phenotype | 3.1.7 | A custom phenotype (TBD) specification is used to identify the patient to access MyPAIN | Custom Phenotype + trigger | Core | CPT codes for relevant procedures |
| Phenotype | 3.1.8 | A custom phenotype (TBD) specification is used to identify the patient to access MyPAIN | Custom Phenotype + trigger | Core | Target practice sites |
The system to be tested is built using Health Level Seven (HL7) FHIR as the framework for enabling interoperability among the systems, services, and applications involved. From the perspective of the SMART on FHIR applications, all the interactions with the clinical system will be described by and performed through FHIR Application Programming Interfaces (APIs) exposed as industry standard web services. The technical standards on which this project is based are documented in the following references:
Within MyPAIN, data are collected as a QuestionnaireResponse FHIR resource. Specifically, the answers to assessment questions will be recorded in the appropriate elements of a QuestionnaireResponse and posted to each site’s FHIR server. Each site will be responsible for storing the answers provided as part of that QuestionnaireResponse. This storage may be in the form of an additional repository at the site for storing QuestionnaireResponses directly, or it may involve transforming the responses in the questionnaire into observational data that can be stored directly within the EHR through proprietary API calls. Sites have the flexibility to choose the approach they take so long as the interfaces exposed for use by MyPAIN support the required functionality.
Patient: Patient-specific data elements needed to support MyPAIN are already available via the standard EHR FHIR API.
Questionnaire: Questionnaire-specific data elements needed to support MyPAIN are NOT already available via the standard EHR FHIR API nor do these data elements specifically exist as standards-based shareable resources in the EHR. Many EHRs contain resources relevant to the questionnaire data element but these are not well documented or understood from a shareability standpoint.
Within PainManager, input data may consist of the results of the SDM encounter, captured as a clinical note and posted to the EHR’s FHIR Server. The sites will be responsible for storing the resulting clinical note, either as a native capability of the EHR’s existing FHIR server or as additional functionality exposed as an FHIR API through the Site-Specific Adapter.
Patient: Patient-specific data elements needed to support MyPAIN are already available via the standard EHR FHIR API.
Within the Phenotype/Trigger, the system must be able to capture whether a patient has been selected as a candidate for MyPAIN. In addition, the system will need to be able to determine the current status of the invitation to MyPAIN (either by sending on a preconfigured interval prior to a scheduled encounter or by specifically recording MyPAIN invitation status for selected patients). Although this aspect is managed by the sites, the data about whether a patient is enrolled in MyPAIN, and the assessment data gathered from their MyPAIN session(s), need to be available through the FHIR API used by the MyPAIN and PainManager applications.
Testing roles: The two central classes of testing roles are (1) artifact developers and (2) site developers/implementers. These testing roles are further outlined below.
User Roles: The system’s two central classes are (1) patients and their families/caregivers and (2) clinicians. These and other potential user classes and their characteristics are outlined in more detail below.
The MyPAIN application will interact with the EHR through a FHIR API conforming to the Structured Data Capture implementation guide (HL7FHIR, 2018c) and specifically the Form Filler role defined within the implementation guide.
The CDS4CPM Implementation Guide will describe Questionnaire resources that capture the assessment questions displayed as part of the MyPAIN application, and this questionnaire will provide the terminologies to be used to record the assessment information, specifically any LOINC codes associated with the PROMIS-based questions and any site-specific codes that support the exchange of data not identifiable with existing standard terminologies.
In addition, the MyPAIN application will display basic patient demographic information for the purposes of confirming the patient interaction. The existing EHR FHIR APIs support this information through the Patient resource, and specifically the USCore Patient profile (HL7FHIR, 2018c).
For the sequence diagrams which follow, we are including both common and site level implementation details (and associated testing discussions), however, in the final implementation, the site specific details may diverge slightly.
Below is a sequence diagram for the MyPAIN assessment.
The PainManager application will interact with the EHR through a FHIR API conforming to the US Core Profiles, as described in the system design and data design sections of this document.
The CDS4CPM Implementation Guide will nominate these profiles and provide specific guidance for implementing sites to support. This support can be met through either existing FHIR API functionality provided by the EHR’s FHIR Server, or it can be built up and exposed as part of the site-specific adapter.
PainManager can also be configured to use CDS Hooks as a mechanism to provide a recommendation to the clinician that a patient is either enrolled in MyPAIN, or has MyPAIN assessment data available, or both. While both options will be fully supported in the final artifact, this ‘configuration option’ would require some support of the CDS Hooks service within the FHIR façade.
In addition, PainManager can be configured to use a PDMP feed to retrieve patient-specific dispensing medication information. For this capability, the PainManager application will expect to access an FHIR API conforming to the ONC US Medication Profiles (HL7FHIR, 2018c), specifically the PDMP Responder role defined within that implementation guide.
Below is a sequence diagram for the SDM encounter supported by PainManager.
Exhibit 6 - PainManager Shared Decision Making (SDM) Encounter
Indication of enrollment in MyPAIN, invitation of enrolled patients through the patient portal, and management of the status of invitations must be provided by implementing sites, since no standard approach exists that can support these capabilities.
There may be a need to align the delivery of messaging (e.g., to a patient from the EHR patient portal or to a provider from the portal or similar) to the best method available at each site. For example, messaging patients through the existing appointment reminder system could be beneficial; this would then point the patient to MyPAIN in their patient portal application. Details on this are to be determined by the implementing site.
Exhibits 7 and 8 show a sequence diagram for the phenotype and trigger process.
Exhibit 7 - Patient Cohort Management and Notification
Exhibit 8 - MyPAIN Data Notification
Throughout this Testing Plan reference has been made to relevant Site-specific Adapters and a related FHIR Façade. This container is conceptual and requires components to be built on both sides of the system—the standards-based artifact side builds standards-based requests or posts which get handed off to either FHIR servers or to appropriate site-specific adapters to fulfill these requests or posts. This means that the core artifact development work and subsequent Testing Plan go as far as the standards allow and the sites must develop solutions that can handle such requests. During the build phase we will continue to define interface details. To the greatest extent possible, the details of these site-specific adapters and any associated testing will be included in the final IG.
The developer is responsible for all baseline testing and will provide guidance and support in the development of an infrastructure for sites to commence feature and function-level integration testing. This infrastructure will also support a continuous release cycle to maximize the opportunity to share updates seamlessly from developer to site and from site to site whenever possible.
The development team can develop unit tests involving edge cases and test accordingly. This verifies basic functionality as we understand it but this still needs validation from a clinical perspective. Sites will provide the clinical support needed to test effectively. This may include the development of testing scenarios and/or test data using clinical input from the project team or institutional SMEs. This includes ensuring that clinical staff are available and attendant to the intended clinical goals of the system. In support of this activity, sites will also develop relevant test data sets including the user stories and edge cases that will facilitate robust and complete testing scenarios.
In terms of the management of test data both where it originates and who is responsible for using which test set, these determinations will be outlined in a future version of this Testing Plan. The use of stories to support development and to help prioritize the work in sprints may be repurposed to aid in the development of user stories for the development of relevant test data that addresses core functionality and edge cases where real data may not represent what is expected in the reference implementation environment. These efforts will also inform the implementation guide.
Below, the strategies and procedures for testing the CDS4CPM system are outlined in more detail. The elements described include the overall development and testing environment, testing procedures, the sequencing of the stages of testing, and plans for the identification and/or development of testing data.
The Atlassian software tool Jira will be used throughout the development process to manage the overall waterfall approach with agile sprints embedded in the timeline. The waterfall allows the team to project out and estimate the work ahead and aim for a target deliverable date. The agile process within this waterfall approach supports iterating through different types (from proprietary to fully standards based) of approaches. This process will be coupled with an agile approach to testing to help ensure that both site-level coordination and iterative testing are featured throughout the development process. Testing phases may typically fall in a specific order and may also be repeated or cycled through as needed to reach identified targets or benchmarks.
Below, the six testing phases are outlined in some detail. These include logic testing, patient population testing, batch and unit testing, end-to-end testing, integration and performance testing (to include any regression testing), and user acceptance testing (UAT). Plans regarding the acquisition or development of relevant test data are also described.
The existing Pain Management Summary references key Centers for Disease Control and Prevention CDC guidelines (3, 8, 10 and 11) for the implementation of specific logic components. The logic testing step will help ensure that the CQL developed to support this logic, which will be substantially revised to reflect updates to the Opioid IG, are functioning as expected in PainManager.
Similarly, the existing MME/Maximum Equivalent Daily Dose (MEDD) calculator based on CDC guideline #5 wwill be updated to be leveraged by PainManager to deliver an MEDD average based on either the currently calculated MEDD or the line level medication data supplied in the FHIR facade. To determine the best approach, clinical review of the performance of the calculator will be compared against the EHR calculated value. This custom-built logic testing process will allow the development team to make adjustments to the calculator and the project team to make a decision about the optimal implementation solution for the planned pilots.
Additional logic testing will be conducted as needed.
Plans for the initial phases of patient population testing will involve the use of synthetic patient data from a service like Synthea. This use of synthetic data will facilitate testing with an approximation of real patient data, which can help with some aspects of more realistic testing but will lack the inconsistencies and site-specific preferences or limitations of more realistic data.
There are some options at the site level to use or develop more complete and realistic patient test data. Steps toward developing or accessing this data will be detailed further in the next version of the Testing Plan.
During the sprint planning process, the development team will identify relevant ‘batches’ and ‘units’ (or aggregates of batches) for testing. Not all sprints will result in a testable batch and not all batches or units will represent components or containers to test. However, as the work progresses, some specificity regarding a ‘testable batch’ or ‘testable unit’ will be defined and the testing process for those elements will be described and executed. The goal is to both execute testing at the smallest discrete level for a batch as is reasonable so that the development process benefits from that level of transparency when testing and to provide insight for the sites on how to emulate this process when they receive items to test.
Without any discrete batches to test at the time of writing this plan, the specification of a batch and associated process of testing is still to be determined. Additionally, the specifics of site handoff for testing will need to be detailed in a future version of this Testing Plan.
As the waterfall model provides all three development teams with some insight into how items are expected to be released and when, each team is working to develop an infrastructure that can support testing, development to manage the integration, and ultimately the hosting environment needed to implement the system. This infrastructure will benefit from smoke testing at the “hello world” level, which will confirm that the necessary services are in place and that they can perform a rudimentary “handshake” to establish communication. The basic concept is that by “standing up” the HAPI FHIR environment and testing connections between the EHR, the FHIR server, and some limited implementation of the artifacts (the system), the three development teams can ensure that the infrastructure is in place to begin development and testing in earnest with some confidence that things should work.
This phase of testing will be repeated once the final FHIR façade is in place and the artifacts have met the MVP stage. More details on the MVP product and its related data elements and functionality will be provided in a forthcoming version of this Testing Plan.
Combining smoke testing with multiple batches or units represents the movement to integration testing. At this stage, development components and then containers will be tested in their intended integration environments. There may still be significant parts of the overall system missing but multiple pieces will be ready to incorporate and connections to the intended environment for the final deployment of the system are required for this phase of testing. At this stage, we will put in place specific performance benchmarks. The use case or user story guiding overall development will be used to establish those benchmarks, and detailed data on testing success will be captured and evaluated to determine next steps.
Some initial UAT testing from the perspective of the patient or provider user is important to incorporate early. While we may not formally engage an expected end user, there will be a need to assume some level of engagement on UAT early and then throughout the process. The project will leverage members at the sites to successfully plan this out and engage relevant stakeholders. More details surrounding the final phases of testing to include UAT will be outlined in a future version of this Testing Plan.
As mentioned in Section 4.3, some effort will need to be dedicated to the identification or development of testing data that can support the full range of testing required to fully execute this Testing Plan. Between synthetic patient data, site-level test data, and developed testing data (e.g., from de-identified real patient data), plans are being developed to ensure that the full range of testing can be supported with relevant patient data. The details of creation and use of test data will be provided in a forthcoming version of this Testing Plan.
Key members of the development team have worked intensively to develop a detailed timeline of the steps and activities that are required to support the core and site builds. Appendix A provides a working version of the full project timeline, including a GANTT chart timeline. Using Smartsheet, RTI is working to align the schedule of activities submitted by the teams at Alphora, VUMC, and UCM to identify points of handoff and when various items will be available for testing. Below is a detailed breakout of the overall timeline which highlights (in green) areas in which we expect testing to take place.
At a high level, VUMC will begin developing the FHIR façade and will make components available to UCM in an agile fashion to test (this work has already begun). Meanwhile, both sites will be working to develop the environments needed for the site-specific adapter development work. Both sites aim to achieve an initial “smoke test” to verify basic connectivity with stub versions of PainManager and MyPAIN by mid-August, when the MVP versions of these applications will become available through Alphora. Through August, September, and October, sites will continue to test components and containers. Full testing of the PainManager application is expected in September, and of the MyPAIN application in October. By late October and into November, sites will be expected to support end-to-end integrated testing of these two applications. Exhibit 9 provides the detailed activities and initial date expectations provided to RTI, which will be refined, aligned, and developed into sprints during the build and testing process.
The focus of this version of the Testing Plan is the core standards-based artifacts (the CDS4CPM system). Although some aspects of the site-specific integration are described in this document, the bulk of the testing details for that phase of development and implementation will be presented in a future version of the Testing Plan.
[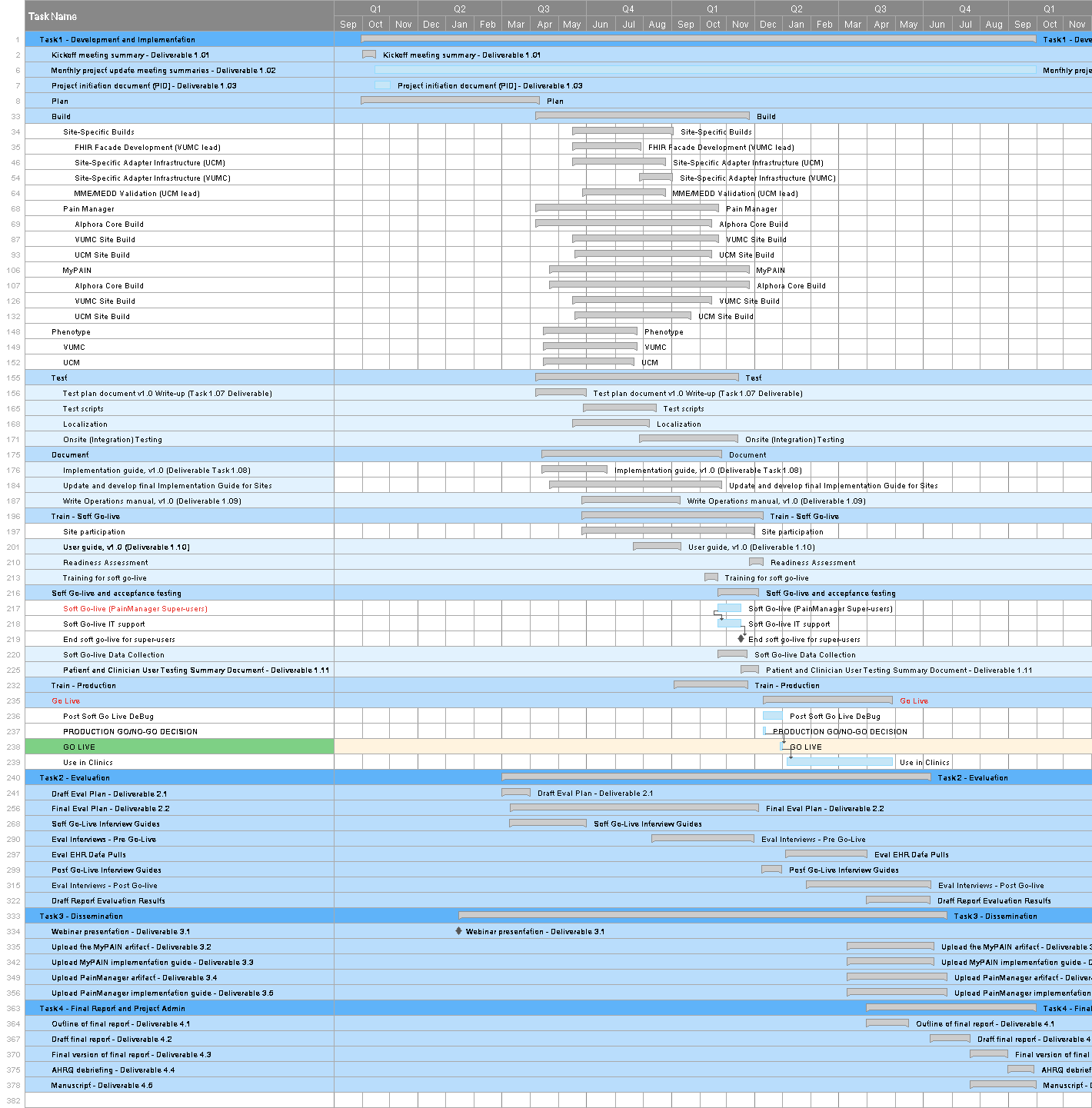 ](assets/images/Full_schedule_pt1.png
](assets/images/Full_schedule_pt1.png
[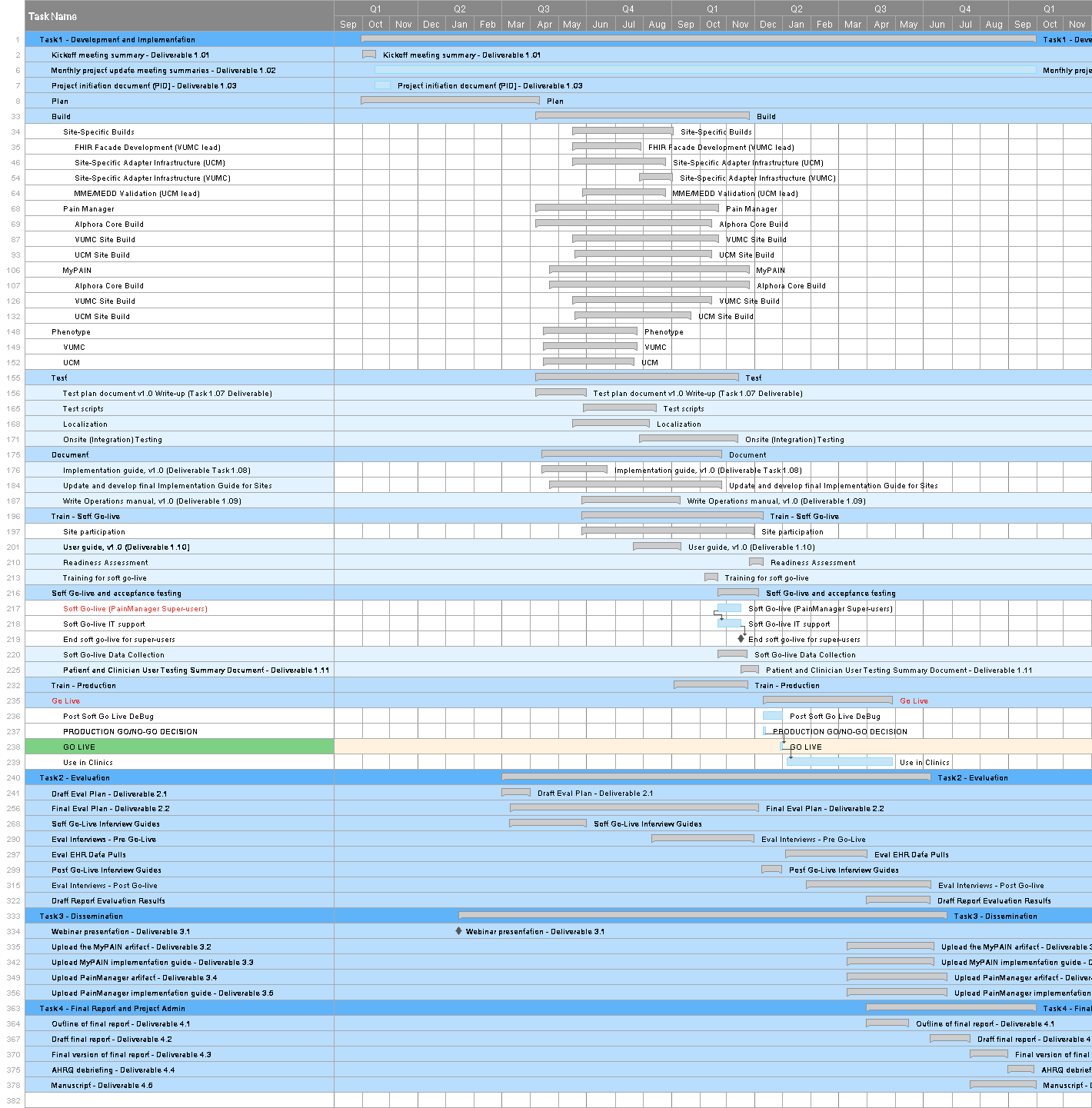 ](assets/images/Full_schedule_pt2.png
](assets/images/Full_schedule_pt2.png