CH ELM (R4)
1.7.0-ci-build - ci-build
CH ELM (R4)
1.7.0-ci-build - ci-build
CH ELM (R4), published by Federal Office of Public Health FOPH. This guide is not an authorized publication; it is the continuous build for version 1.7.0-ci-build built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/ahdis/ch-elm/ and changes regularly. See the Directory of published versions
The website of the Federal Office of Public Health (FOPH) (EN, DE, FR, IT) provides further documentation on infectious diseases requiring notification. The most important points for the implementation of this FHIR exchange format are included here.
Please visit the FOPH website to access the most recent documents in the desired language (EN, DE, FR, IT) for each topic.
The reporting obligation is the key systemic element for the surveillance of communicable diseases in Switzerland. Notifiable laboratory findings must comply with the legal provisions regarding reporting criteria, reporting deadlines and reporting data in accordance with the Ordinance of the FDHA on the Reporting of Observations of Communicable human diseases (SR 818.101.126) (DE, FR, IT).
Depending on the organism (leading code), the requirement for how the patient’s personal data, such as name or address, (e.g. Ernst Karl Tanner, Tannenstrasse 10a, 3000 Bern) is reported varies. There are different patient schemas to be used:
The complete overview of which characteristics are used for which organisms can be found in the ConceptMap CH ELM Results To FOPH Patient Name Representation and in the Ordinance of the FDHA on the Reporting of Observations of Communicable human diseases (SR 818.101.126) (DE, FR, IT).
The laboratory report is currently either of the type organism detection (LOINC 18725-2 Microbiology studies (set)) or resistance detection (LOINC 18769-0 Microbial susceptibility tests Set). These types are defined in the ValueSet CH ELM Lab Study Types and are represented in the Composition.section.code element of the respective document.
Composition.section.code = LOINC 18725-2 Microbiology studies (set)Observation.value = Positive/Negative (CH ELM Results Coded Values Laboratory)Observation.interpretation = Positive/Negative (CH ELM Interpretation Codes Positive and Negative)Example: Laboratory report for Neisseria gonorrhoeae
Composition.section.code = LOINC 18769-0 Microbial susceptibility tests SetObservation.interpretation = Resistant/Susceptible (CH ELM Interpretation Codes Resistant and Susceptible)The performed laboratory test is represented by a so called leading code from the valueset ValueSet CH ELM Results Laboratory Observation containing codes from the LOINC and SNOMED codesystem. The leading code reflects a 4-axis model and laboratories are requested to choose the code that corresponds to the specific parameters of the performed laboratory test. Ideally, the chosen code covers all 4 axes.
Example Neisseria gonorrhoeae: The leading LOINC code 697-3 Neisseria gonorrhoeae [Presence] in Urethra by Organism specific culture covering all 4 axes:
Note: The Specimen.type.text element in this case contains a fixed text as value: “Material declared by Observation.code or non-mandatory”.
Important note:
The ValueSet CH ELM Results Laboratory Observation is a selection of LOINC or SNOMED codes related to notifiable diseases and their legal basis. The ValueSet can be adapted according to laboratory-specific needs - please contact the FOPH in this regard.
If the leading code does not cover all axes, the missing axis must be completed by an additional code.
In some cases, the collection material must be explicitly specified.
Example Chlamydia trachomatis: The leading code (Observation.code = LOINC 6349-5) is completed by an additional code for the collection material (Specimen.type = SNOMED CT 119393003).
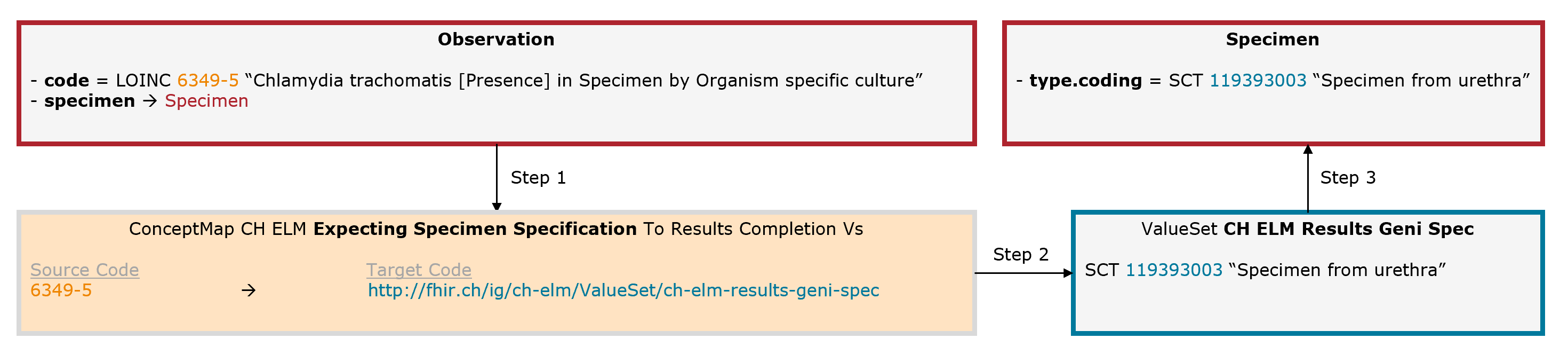
Fig. 7: Schematic illustration of the mechanism for the expecting specimen specification (for simplicity, only the relevant elements are shown)
In some cases, an additional organism must be specified.
Example Carbapenemase-producing Enterobacteriaceae (CPE): The leading code (Observation.code = LOINC 85827-4) is completed by an additional code for the organism (Observation.valueCodeableConcept = SNOMED CT 56415008).
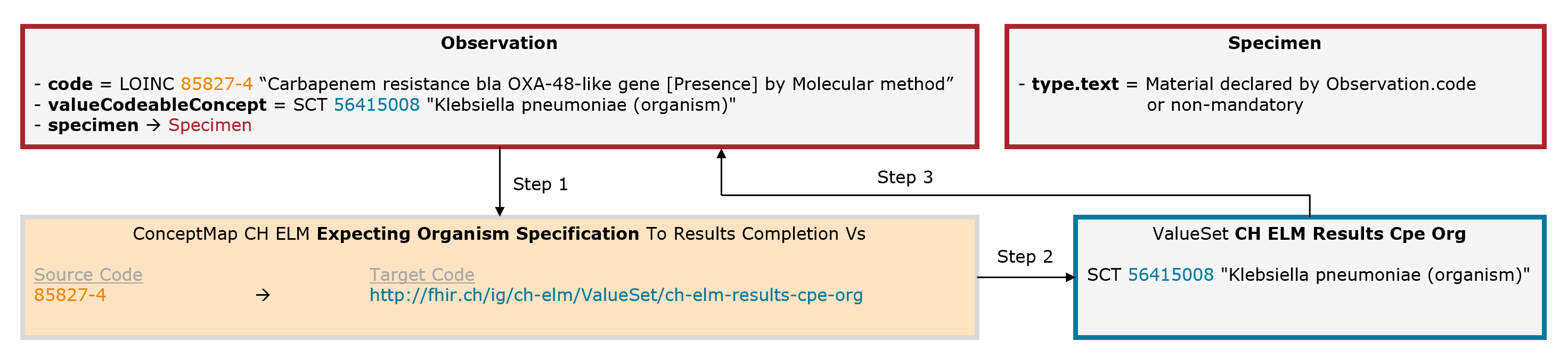
Fig. 8: Schematic illustration of the mechanism for the expecting organism specification (for simplicity, only the relevant elements are shown)
Depending on the leading code different interpretation codes are allowed. The ConceptMap specifies which values from which ValueSet are allowed (e.g. for Neisseria gonorrhoeae the ValueSet: CH ELM Interpretation Codes Positive is specified, which allows only a positive interpretation code to be specified).
In cases where certain test results like physical quantities, sequencing-/typing results etc. or a series of test values with their associated measuring units and interpretations are to be transmitted, a specific observation profile is expected depending on the leading code. The ConceptMap defines which profile has to be considered.
The exchange format defines the FHIR document for reporting to the FOPH so that one document per organism per patient is submitted.