Supply of Products for Healthcare (SUPPLY)
0.3.0 - ci-build
Supply of Products for Healthcare (SUPPLY)
0.3.0 - ci-build
Supply of Products for Healthcare (SUPPLY), published by IHE Pharmacy Technical Committee. This guide is not an authorized publication; it is the continuous build for version 0.3.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/IHE/pharm-supply/ and changes regularly. See the Directory of published versions
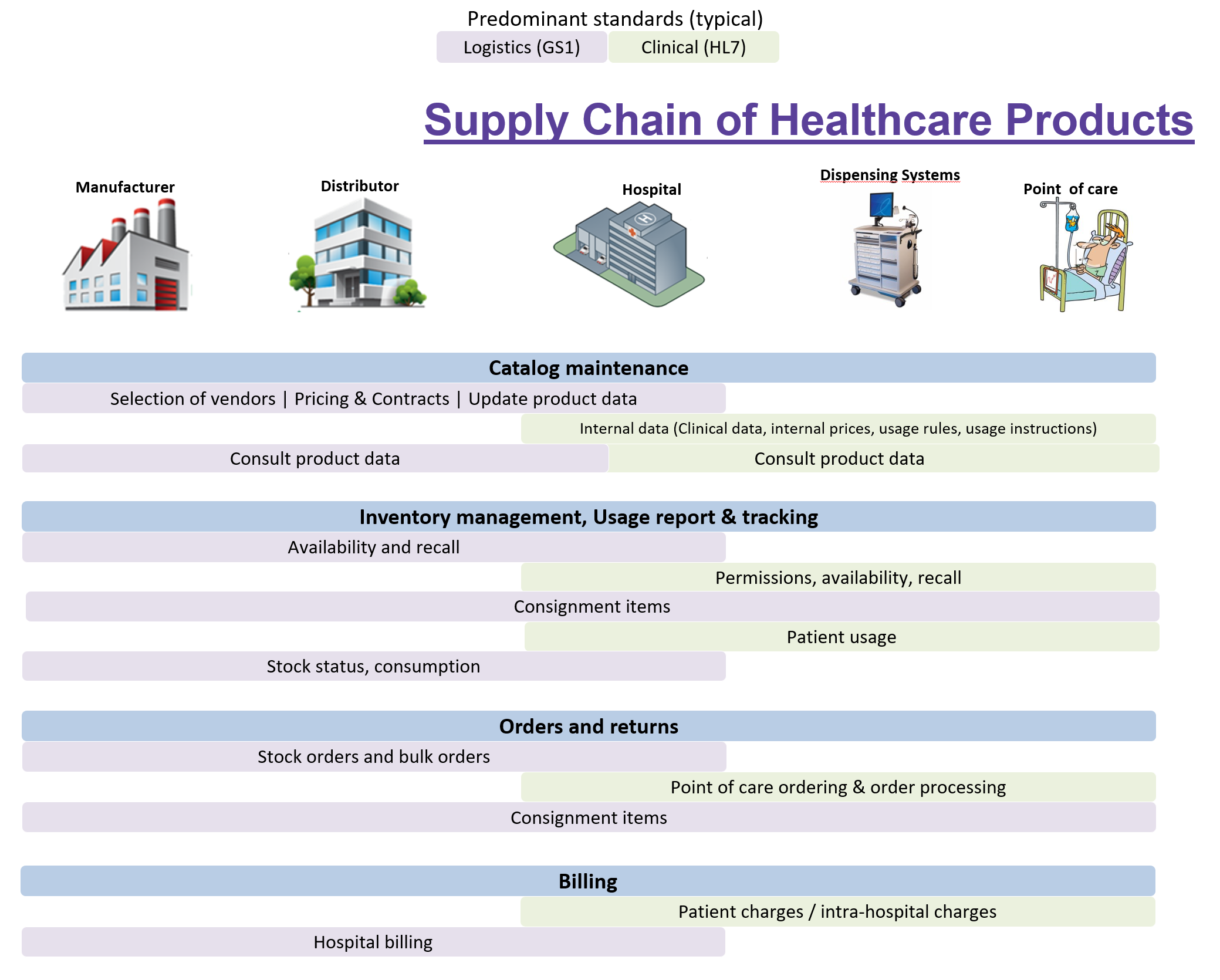
IHE provides a set of standard mechanisms for handling the key aspects of supplying and delivering healthcare products.
…
Supply chain topologies are business-driven and vary considerably. The IHE profiles do not intend to constrain that variability, rather support it by providing stackable components - actors and transactions - for interoperability across the key functions. This means that the scope is
The scope covers
…
…
This IHE specification …
There are three key types of standards that apply to the use cases described:
Data Exchange specifications – these cover the messages between systems.
Examples:
– HL7®1 version 2 – a message-oriented standard with wide global adoption
– CDA®2 and Version 3 – structured content, document-oriented with global recognition and implementations e.g., mmany IHE profiles
– FHIR®3 – multi-paradigm standard for healthcare data exchange, supporting messages, documents, and in multiple formats, e.g., XML or JSON.
– GS1 XML – Supply-chain dedicated standard, based on XML documents, with global implementation in several industries and in the healthcare supply chain.
Terminologies and identification codes – standards that provide reference values for use by the different actors. For example, GTIN (Global Trade Item Number) and other GS1 keys, IDMP (Identification of Medicinal Products), or any national health product terminology.
AIDC – standards for encoding information in computer-readable form like barcodes. Examples are barcodes generated by GS1 (traditional and 2D), HIBCC or ICCBBA.
The different standards have a preferred applicability to different purposes. For example, blood products and medical devices use different standards for barcodes; HL7 is usually more dedicated to data exchange inside an institution, while GS1 and x12 usually handle the “outside” logistics.
The following table shows the available standards and their match to the main requirements identified. This is not an exhaustive analysis, rather an indication of maturity, coverage and adequacy.
The standards analyzed are selected from common implementations and SDOs actively working on this topic, and for the most critical transactions identified:
| HL7 v2 (v2.9.x) | HL7 FHIR | GS1 XML | x12 | UBL | |
|---|---|---|---|---|---|
| Supply Request | Yes | Yes4 | Yes | Yes | |
| Supply Delivery | Yes | Yes | Yes | Yes | |
| Recall | Unknown | Unknown5 | Yes | Yes | |
| Inventory status | Unknown | Unknown | Yes | Yes | |
| Consumption | Unknown | Unknown | Yes | Not found | |
| Product Discovery | Yes (basic) | Partial (e.g., IHE UBP profile) | Yes | Yes |
These standards have different applicability in different areas. Traditionally, the supply chain has been focused on supply-specific aspects which are then adapted to healthcare needs – which brings a number of advantages, namely the “harmonization” of healthcare needs with common good practices for supply, like traceability, flexibility, etc.
This alignment requires collaboration by implementers and also by standards organizations. One example of such collaboration is the Memo of Understanding between GS1 and HL7, establishing the intention to contribute to the safety and integrity of the supply chain: http://www.hl7.org/documentcenter/public_temp_AA12D9B2-1C23-BA17-0C7AC26D7769A523/pressreleases/HL7_PRESS_20131014.pdf
GS1 has introduced the GS1 Digital Link standard – a modern, web-based standard for accessing the information while reusing the existing (and evolving) GS1 models.
The list of standards in the previous table is not an exhaustive list of applicable or implemented standards. It is a shortlist with the standards that at the time of producing this paper, have been found to be mature and commonly used in the Supply of Medical products.
Standards like GS1 and X12 are commonly used industry standards.
For healthcare, HL7 v2, HL7 FHIR and GS1 have a broad acceptance by suppliers and users.
For this reason, we mention the standards in the overview but this document uses HL7 and GS1 standards to describe the use cases. This is also an indication that HL7 and GS1 standards, given their functional compatibility with the model described and with each other, are good candidates for IHE profiles where we want to maintain the continuum of information throughout the Supply Chain.
HL7 is the registered trademark of Health Level Seven International. ↩
CDA is the registered trademark of Health Level Seven International. ↩
FHIR is the registered trademark of Health Level Seven International. ↩
HL7 FHIR has resources for Supply Request and Delivery. At the time of writing of this document, these resources have a Maturity Level 1, which means these resources may still evolve. ↩
There is currently no FHIR resource or recommendation covering Recall, Inventory Status, Consumption. ↩