Computable Care Guidelines
1.0.1-current - ci-build
Computable Care Guidelines
1.0.1-current - ci-build
Computable Care Guidelines, published by IHE QRPH Technical Committee. This guide is not an authorized publication; it is the continuous build for version 1.0.1-current built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/IHE/QRPH.CCG/ and changes regularly. See the Directory of published versions
The IHE Computable Care Guidelines (CCG) specification describes the following key things:
It describes the digital health system actors that would participate in an end-to-end CCG ecosystem capable of supporting the defined CCG use cases (listed below).
It defines a normative grammar for expressing guideline-based care recommendations that can be consistently and reliably processed and operationalized by conformant digital health software solutions across the whole of the care delivery network.
It defines a normative pattern for processing CCG recommendations that will ensure multiple CCGs (e.g., for multiple health conditions) can be concurrently executed and that all relevant care recommendations will be proposed to the end-user in support of their care decision-making.
It documents a base health data content model that provides all ecosystem actors with a common underlying framework for defining, sharing, and executing CCG artifacts. It is anticipated that implementing jurisdictions may replace this base model with their national or regional continuity of care content specifications.
The digital health ecosystem of CCG actors and transactions is designed to satisfy four use cases:
UC-1: A person who is a guideline author uses a digital health tool (which plays the role of a Guideline Publisher) to retrieve and edit an existing CCG or to create a brand-new CCG (L3)1 artifact. Maybe she digitally signs the artifact (at the top level, or every recommendation). She then publishes the CCG (L3) artifact to a Guideline Repository.
UC-2: A digital health solution that executes CCGs (e.g., a Guideline Engine) refreshes its local cache of CCGs from a Guideline Repository.
UC-3: A person who is a care provider leverages her digital health solution (e.g., an EMR) to associate a patient with one or more CCG-supported, evidence-based care programs.
UC-4: A person who is a care provider has an ambulatory encounter with a patient who is enrolled in one or more CCG-supported care plans. During the encounter, she leverages her digital health solution (e.g., EMR, playing the role of a Guideline Performer) to invoke processing by a Guideline Engine to concurrently execute the relevant CCGs. She acts upon the recommendations to provide person-centric, guideline-adherent care.
Countries are increasingly utilizing digital technology that leverages person-centric health data to facilitate scale-up of public health interventions; to improve client-level care; and to ensure accountability at all levels of the health system. However, the transformative value of these digital systems has not yet been fully realized.
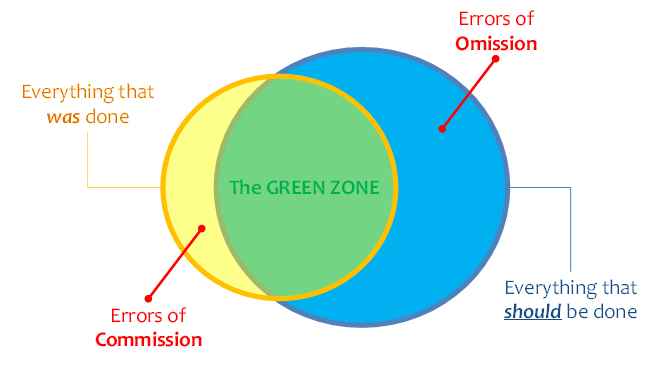
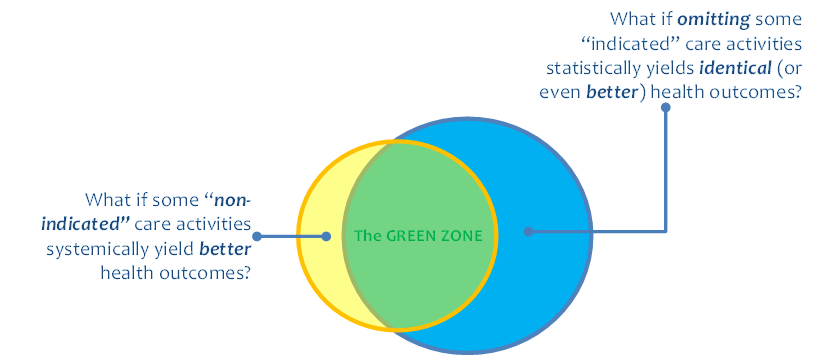
We are better and better able to collect information about what happened on a client’s health journey. This can be illustrated, graphically, by the yellow circle in Figure 1. However, we do not yet (today) have a way to broadly share a machine-processable description of what should happen (the blue circle in Figure 1) and to leverage digital health investments to systemically close the “know-do gap” between what was done and what should have been done (illustrated by the “green zone” in the figure). A machine-processable description of what should happen could be called a computable care guideline, or CCG.
Closing the know-do gap will enable us to reap the significant health outcome improvements that accrue from stronger guideline adherence. As may be noted in Figure 1, evidence shows that errors of omission greatly outnumber errors of commission. These are difficult errors to catch and to address. But by describing what should happen, CCGs afford us way to treat the absence of a signal as a signal.
Broadly scaling CCGs, however, faces a set of key challenges:
To support patients with co-morbidities, multiple CCG artifacts must be concurrently executable.
To ensure interoperability across entire care networks, CCGs must be based on a common data model and these data must be readily available at the point of service.
In the interests of patient safety, CCG actors must ensure that all relevant care recommendations are presented to a practitioner during a care encounter; nothing can be inadvertently missed during concurrent execution of multiple CCGs.
To meet adherence to relevant software-as-a-medical-device (SaMD) regulations, CCG artifacts and transaction processing workflows must be conformance-testable.
Significant progress has been made by HL7 and IHE as collaborators on the CCG Gemini Project. The HL7 CPG-on-FHIR IG2 (Implementation Guide) has been developed and has completed multiple ballots. This HL7 IG describes how a CCG may be defined using the FHIR standard. In a complementary way, the role of the IHE CCG Profile is to leverage this underlying HL7 specification and to constrain it to address the challenges, as noted above, associated with broadly scaling conformance-testable, concurrently executable CCGs in both OECD3 and LMIC4 contexts.
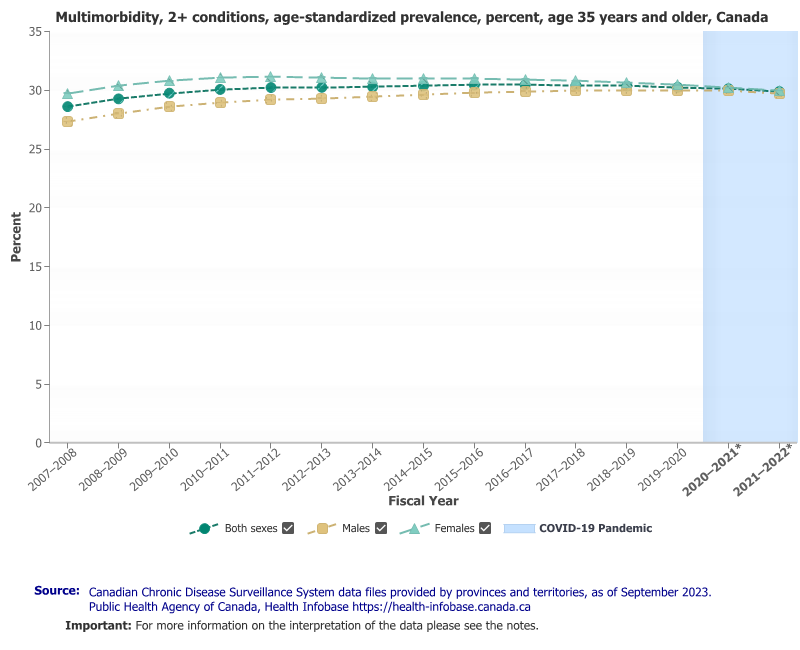
Improving the care continuity and care quality, especially for co-morbid patients with multiple chronic conditions, addresses a big and expensive problem. In Canada, for example, 73% of persons over 65 years of age suffer from at least one chronic condition5, and 30% of Canadians over 35 years of age suffer two or more conditions (note Figure 2). It is estimated the burden of chronic disease represents a cost to the Canadian economy of more than $190 billion (CAD) annually6 and that the direct costs of chronic disease management account for 58% of Canada’s total healthcare spend.7
“Health care in all global settings today suffers from high levels of defects in quality across many domains, and this poor-quality care causes ongoing damage to human health. Hospitalizations in low- and middle-income countries (LMICs) lead to 134 million adverse events each year, and these adverse events contribute to more than 2.5 million deaths annually. More than 830 million people with a diagnosed noncommunicable disease (NCD) are not being treated, and more than 4 million avoidable quality-related deaths each year are attributable to ineffective care for NCDs. In total, between 5.7 and 8.4 million deaths occur annually from poor quality of care in LMICs for the selected set of conditions the committee analyzed… which represents between 10 and 15 percent of the total deaths in LMICs reported by the World Health Organization (WHO) in 2015. For some conditions, deaths due to poor quality contribute to more than half of overall deaths.”8
A 2018 care quality report was jointly published by the OECD, WHO, and World Bank9. Among the report’s findings:
One in ten patients is harmed during medical treatment in high income countries.
Health care workers in seven low- and middle-income African countries were only able to make accurate diagnoses one third to three quarters of the time, and clinical guidelines for common conditions were followed less than 45 percent of the time on average.
Research in eight high-mortality countries in the Caribbean and Africa found that effective, quality maternal and child health services are far less prevalent than suggested by just looking at access to services. For example, just 28 percent of antenatal care, 26 percent of family planning services and 21 percent of sick-child care across these countries qualified as ‘effective.’
Around 15 percent of hospital expenditure in high-income countries is due to mistakes in care or patients being infected while in hospitals.
The successful work of the underlying HL7 CPG-on-FHIR IG has been constrained to address key implementation issues in-scope for the IHE CCG Profile. A strategy of constraining optionality has been adopted by multiple jurisdictions in their clinical decision support regulatory frameworks. As an example, the US ASTP/ONC has published guidance regarding the methods for conformance-testing digital health solutions that provide Evidence-based Decision Support Interventions (DSI). It stipulates that evidence-based DSIs shall leverage a constrained common data set defined in the US Core Data for Interoperability10. It can also be noted that, under this ONC regulation, a software solution provider must provide an attestation related to the source content, including bibliographic citations of the clinical research of narrative (L1) care guidelines, that inform the DSI's recommendations. Similarly, NHS Scotland has adopted a single-hosted-service approach that constrains optionality and ensures consistency of data and logic execution. This hosted service is ISO-13485 certified to meet relevant Software as a Medical Device (SaMD) regulations. In Denmark, the adoption of a common shared health record has enabled four DSIs to be taken to national scale (see Figure 3). Research on the Danish experience has noted the importance of testability as a key element of success.
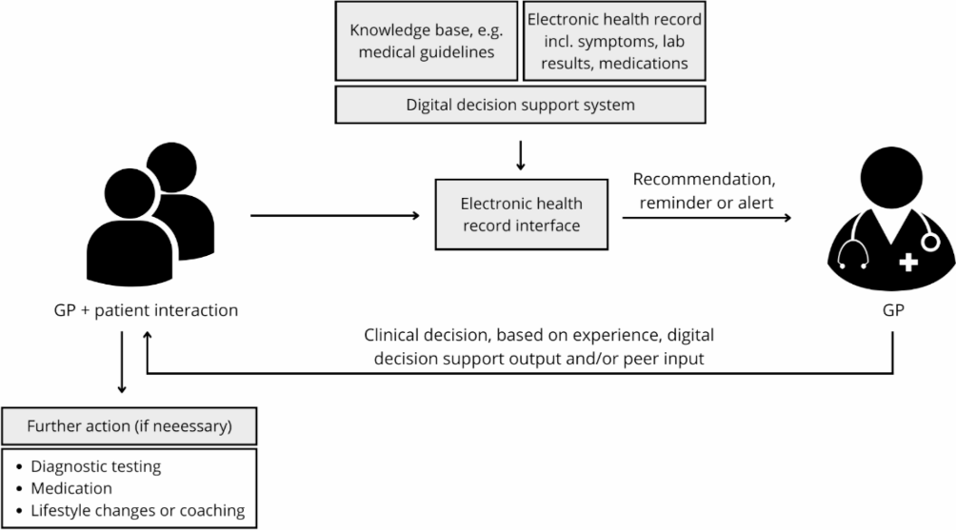
Informed by these examples and others, the IHE CCG Profile constrains the content that may be employed to define CCG logic. The (optional) base CCG content specification is defined in Volume 3 of this profile. In this base content specification, an approach is favored that allows care logic to take into account both patient health data and contextual attributes of the care encounter:
In the base specification, person-centric content is defined in terms of the IHE International Patient Summary (IPS) Profile (Complete Option). The Complete Option stipulates that optional sections of an IPS document be instead treated as “required if known”. In this way, properly-coded content that is collected as part of a CCG-supported care encounter will always be available to drive care logic during any subsequent encounter. This creates a virtuous cycle of ever-improving person-centric health data.
To support evidence-based decision-making that may be driven by the care context (facility’s geography, available services, provider, provider scope of practice, etc.), the Encounter resource and the content specified by the IHE mobile Care Services Discovery (mCSD) Profile may also be employed to drive CCG care logic.
It is anticipated that implementing jurisdictions will adopt the base common content model or may define an alternate common content model based on their domestic care continuity specifications (e.g., for the USA: UCSDI, for Canada: PS:CA, for Europe: the European Patient Summary, etc.). An alterative common content model SHALL be defined in a relevant Volume 4 section of the IHE CCG Profile. Each ecosystem actor SHALL declare its support for a content model option. As a practical constraint, all actors in a CCG-conformant ecosystem SHALL support the same common content model option.
IHE's choice of IPS as the foundation of the base common content model is intended to be risk-mitigating. It is noteworthy that many countries (Canada, Sri Lanka, Botswana, New Zealand, etc.) and many multilateral organizations (Asia eHealth Information Network11, OpenHIE Community12, etc.) have adopted or advocated for the adoption of the IPS as the basis for a national health summary specification. The joint EU-USA Trillium II project reported on the significant value of leveraging IPS across multiple CDS-supported use cases.
Included in the base common content model is a normative grammar related to how CCG artifacts are defined. Action research into CCG formats was conducted by a joint team from Canada Health Infoway, Cancer Care Ontario, and Hamilton Health Sciences. A working prototype was demonstrated at the 2020 IHE North American Connectathon. Informed by this research, and to support the concurrent execution of multiple CCGs, a Folder-and-CARDs metaphor is documented in Volume 3. Leveraging this model, one can think of a CCG as a set of care recommendations where each recommendation is described by a CARD. This model is illustrated in Figure 4.
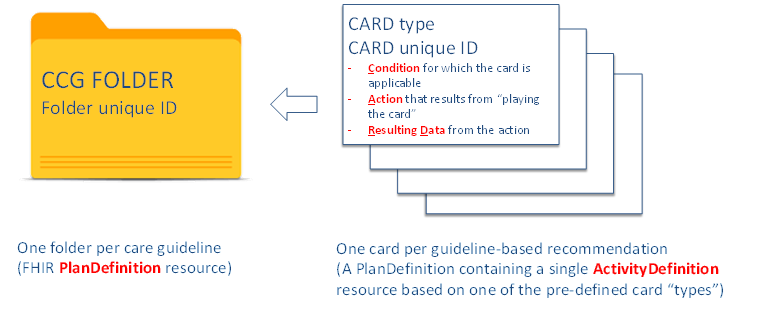
Here, CARD is an acronym. The recommendation is applicable when a set of defined Conditions are met. The recommendation describes an Action that should be taken. The list of possible actions is defined as a subset of the ActivityDefinition “verbs”13 described in the CPG-on-FHIR specification. When the action is taken, there is Resulting Data. All the recommendations for a particular care guideline are stored in that guideline’s Folder. For example, the set of CARDs related to evidence-based Diabetes care would be contained a Diabetes Folder.
In practical terms, this approach supports simplifying both the authoring of CCGs and their subsequent execution.
A uniquely identified CARD is independently defined for each guideline-based recommendation; this definition is expressed using a FHIR PlanDefinition resource containing a single ActivityDefintion resource (from the constrained list of CARD “types”).
The CARD’s PlanDefinition resource declaritively defines the Condition statement(s) that must evaluate to TRUE for the CARD to be applicable. The condition statements’ logic must be expressed in terms of the common content model (either the base defined in Volume 3 or an alternate model documented in Volume 4).
The CARD’s resulting data must also be expressed in terms of the common content model (in Volume 3 or Volume 4).
A CCG Folder is defined using a FHIR PlanDefinition resource. This PlanDefinition will act as a container and reference the full set of relevant CARDs for a particular care plan (e.g., diabetes care, or antenatal care, etc.).
The set of 13 CARD types defined by the base common content model in Volume 3 is listed below:
Provide Information – provide information, counseling, or instructions to the patient.
Collect Information – capture information about the patient.
Request a Service (Lab Order) – create a laboratory / pathology Service Request.
Request a Service (Radiology Order) – create a radiology Service Request.
Request a Service (Procedure Order) – create a procedure Service Request.
Request a Service (Referral) – create a Service Request to refer a patient to another care provider (e.g., perhaps to escalate to a higher level of care).
Propose a Diagnosis – record the patient's diagnosis as a Condition resource.
Order Medication – create a medication order.
Dispense Medication – dispense medications based on an active order.
Administer Medication – administer a dispensed medication.
Request Immunization – create an order for and administer a vaccine.
Stop Activity (Medication Order) - create a Task that, upon being processed, will stop a patient's presently active Medication Order.
Stop Activity (Service Order) - create a Task that, upon being processed, will stop a patient's presently active Service Request.
The Folder-and-CARDs "flattened stack" approach may be contrasted with a “flowchart” approach, as illustrated by Figure 5 and Figure 6. Figure 5 illustrates a flowchart centric approach for describing CCG-supported workflows.
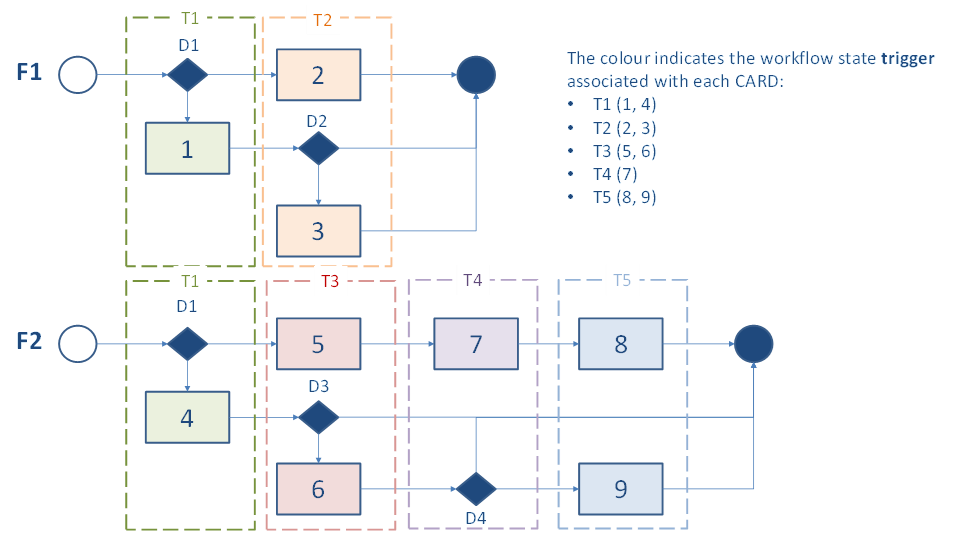
In a flowchart or tree-diagram approach, trigger events are associated with workflow states. This means that for a CARD to evaluate to true, its trigger event must be true and its condition statement must also be true. The trigger events may be leveraged by a digital health solution to inform when it should invoke a CCG evaluation operation ($apply). However, such an approach relies on each individual workflow process being followed in its entirety so that each state in the workflow is reached; otherwise, associated trigger events will not have a chance to fire true and a CARD may be inadvertently missed.

Figure 6 illustrates the “flattened stack” of CARDs resulting from the Folder-and-CARDs content model. For each CARD, the trigger event is omitted and only the condition statements are declaratively defined. Such an approach de-couples the CCG recommendations from the workflow. A CARD fires true when its condition statements are true. This greatly simplifies the concurrent processing of multiple CCGs (e.g., Folders F1 and F2, as illustrated in Figure 5 and Figure 6).
Notionally, we can think of associating a set of relevant Folders with a care subject. During a care encounter, the CARDs from all the relevant Folders are metaphorically shaken out, de-duplicated, and assembled into a single stack. Following is a narrative description related to how concurrent CARD processing may be operationalized; this is not normative, but rather illustrative.
To ensure CARDs are not missed, it is expected that the entire CARD stack is iteratively processed over the course of the encounter. In the first pass, all the CARDs in the stack are processed and those that fire true are processed. As an example, there could be CARDs indicating that, at every encounter, the subject's blood pressure, weight, heart rate, and spirometry measures should be recorded. For each CARD that fired TRUE, there is new resulting data; either the reading is recorded or a data-absent reason is recorded.
This new data is taken into account as the entire stack-of-CARDs is again processed. This is illustrated by the successive invocations of $apply in Figure 6. Based on the blood pressure reading, for example, an “order medications” CARD may fire TRUE during the second pass. As before, either the recommended action is taken, or a reason code is recorded to indicate why the action is not to be taken. This iterative process continues until zero new CARDs fire TRUE, which indicates the processing is DONE.
Of course, the capabilities may be different within different care contexts. Such realities can be reflected in more sophisticated CARD condition logic statements. If a community health worker at a remote health outpost has recorded an elevated blood pressure, for example, it could cause a “care escalation” CARD to fire TRUE. At a subsequent encounter, with a provider who has drug prescribing in their scope of practice, the “order medications” CARD may fire TRUE. It is for this reason that contextual Encounter information is included in the CCG “input” data bundle. These contextual data may be referenced when defining CARD condition statements.
As noted in the introduction, the CCG specification is designed to satisfy four use cases:
UC-1: A person who is a guideline author uses a digital health tool (which plays the role of a Guideline Publisher) to retrieve and edit an existing CCG or to create a brand-new CCG (L3)1 artifact. Maybe she digitally signs the artifact (at the top level, or every recommendation). She then publishes the CCG (L3) artifact to a Guideline Repository.
UC-2: A digital health solution that executes CCGs (a Guideline Engine) refreshes its local cache of CCGs from a Guideline Repository.
UC-3: A person who is a care provider leverages her digital health solution (e.g., an EMR) to associate a patient with one or more CCG-supported, evidence-based care programs.
UC-4: A person who is a care provider has an ambulatory encounter with a patient who is enrolled in one or more CCG-supported care plans. During the encounter, she leverages her digital health solution (e.g., EMR, playing the role of a Guideline Performer) to invoke processing by a Guideline Engine to concurrently execute the relevant CCGs. She acts upon the recommendations to provide person-centric, guideline-adherent care.
To fulfill these use cases, it is expected that the human participants leverage system actors and transactions as defined in the technical sections of this specification. These participants, together, operationalize an ecosystem that supports the patient-safe execution of CCG-supported care encounters, at scale.
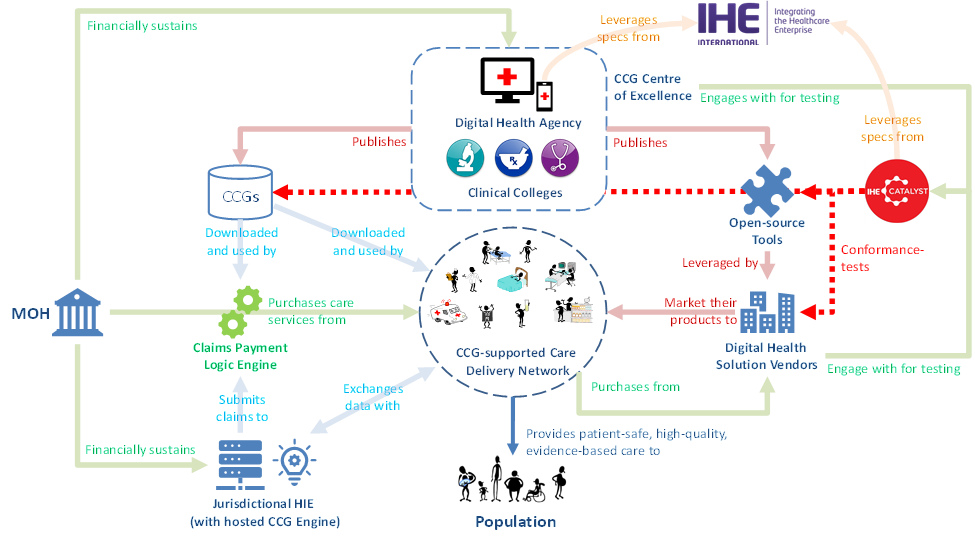
The operation of this ecosystem is pictorially depicted in Figure 7 and Figure 8. From Figure 7 we can note the following:
In use case 1 (UC-1), a Guideline Publisher executes the transaction processing needed to author or update a well-formed CCG artifact. At the successful conclusion of the workflow, the well-formed CCGs are submitted to and ingested and cataloged by a Guideline Repository (State-A).
In UC-2, a Guideline Engine executes the transaction processing needed to refresh its local cache of well-formed CCGs from the content managed by the Guideline Repository. At the successful conclusion of the workflow, the up-to-date CCG artifacts are ingested by the Guideline Engine (State-B) and may be leveraged to support evidence-based care encounters.
In UC-3, a digital health solution (playing the role of a Care Plan Contributor) creates or updates a subject’s Care Plan (persisted and managed by the Care Plan Service). At the successful conclusion of UC-3’s workflow, one or more CCG Folders have been associated with the care subject and are referenced in the subject’s Care Plan (State-C).
The activities relevant to the CCG-supported care encounter are illustrated by Figure 8. From this figure, we can note the following:
The execution of CCG-informed care cannot happen until UC-1, UC-2 and UC-3 have successfully occurred. In some situations, UC-3 may be undertaken as an initial step during the care encounter, itself. Regardless of the timing – State-A, State-B and State-C are pre-conditions to the commencement of UC-4.
The Guideline Performer must be able to establish the data set that reflects the context of the care encounter. This includes up-to-date person-centric health data pertaining to the care subject plus pertinent information related to the encounter context such as relevant information about the care provider, the care location, etc. This initial contextual data must be expressed according to the common data model defined in either Volume 3 or Volume 4 of this IHE CCG Profile (State-D).
During the care encounter, content will be exchanged between the Guideline Performer and Guideline Engine and the engine’s CCG transaction processing will evaluate this content and return appropriate care recommendations. The Guideline Performer will facilitate the actioning of these recommendations (to generate the CARD's Resulting Data) and the iterative evaluation of new content by the Guideline Engine.
At the conclusion, the results of the care encounter are persisted by the Guideline Performer in adherence with the data models defined by either Volume 3 or Volume 4 of this IHE CCG Profile (State-E).
It should be noted that a single digital health solution may group together and incorporate the functionality of both the Guideline Performer and Guideline Engine Actors. Where this is the case, the transaction processing behaviors of this solution represent a black box. In such a scenario, the software solution’s conformance may be established by giving a starting State-D and confirming that ending State-E is achieved.
In the fictitious country of Amalgaland, the Ministry of Health (MOH) has established a CCG Centre of Excellence. The Amalgaland Centre of Excellence (ACE) operates as a partnership between the national Digital Health Agency and the clinical Colleges that, today, publish clinical practice guidelines. The ACE’s role is to leverage these narrative practice guidelines and publish a national set of CCGs. The focus will be on those conditions in Amalgaland’s “top 10” burden of disease (as identified by IHME14) that can be significantly impacted by digital health interventions15.
As a first task, the ACE defines the “style guide” for domestic CCGs; a description of Amalgaland’s national norms and standards for digital health and how this impacts their CCG authoring. Like a growing number of countries, Amalgaland has adopted the FHIR-based International Patient Summary (IPS) as the basis of its national person-centric health data model. The IPS specification has been profiled to reflect Amalgaland’s national code systems. Through its IHE Amalgaland Deployment Committee, the ACE publishes a section in Volume 4 of the IHE IPS Profile, the IHE mCSD Profile, and the IHE CCG Profile that documents its national core data model. This domestic health data model is the basis for CCGs that will be published by the ACE.
Amalgaland’s MOH wishes to leverage its CCG initiative, alongside its national HIE, as part of a larger value-based health services16 (VBHS) agenda. Strategically, a set of five health conditions will be focused on (from the top 10). The ACE will publish CCGs for these conditions, and adherence to these CCGs will be leveraged as part of a revised healthcare provider claims adjudication and payment scheme.
To help ensure its CCGs can be leveraged in this way, the ACE will:
Adopt an internal conformance-testing regime that ensures its CCG artifacts are well-formed and are adherent to the national core data model; and
Digitally sign all its published artifacts.
To be eligible for the VBHS payments scheme, care providers will need to demonstrate:
They are employing a digital health solution that has been conformance-tested by an independent test body as being “CCG-capable”; and
They are leveraging the digitally signed CCGs published by the ACE for each of the five target health conditions; and
For these target conditions, they can use digital health transaction traffic to demonstrate substantial adherence to the evidence-based recommendations across their patient cohorts.
To operationalize its plans, the ACE decides to adopt an open-source CCG authoring tool (a Guideline Publisher, as referenced in Figure 7 and Figure 8). They leverage technical resources within the Digital Health Agency to become source code contributors on the project. They augment the tool with features important to their intended use cases and they develop a national language version of the software.
A set of training materials are developed for the non-technical ACE teammates. This training leverages the GIN McMaster checklist17 and its extension for Computable Guidelines18. It focuses on the adoption, early in the CCG development process, of best practices and precise methods that enable evidence-based recommendations to be expressed in a way that digital health solutions can ingest and operationalize. To bring these best practices to life, the ACE establishes itself as the governance authority of a Guideline Repository operated by the national Digital Health Agency.
To help round out necessary elements of the ecosystem, the Digital Health Agency leverages its internal software team to fork and made available a “conformance-assured” open-source Guideline Engine. This Engine is “pre-configured” to be able to connect to and regularly refresh its CCGs from the Guideline Repository. The Engine’s processing logic follows the MOH’s stipulations regarding digital signatures and the audit trails required to provide evidence of signed-CCG execution. The Engine’s open-source code base is both a teaching tool and a “running start”. It can be used by digital health solution providers in the Amalgaland market to de-risk product development efforts and to accelerate the delivery timeframes for new product releases. As a further ecosystem accelerator, an instance of the Engine is operated by the Digital Health Agency as a shared service that can be securely called by an authenticated digital health solution.
Leveraging its MOH-led approach, Amalgaland ensures that it can establish the key foundational elements needed to support its VBHS agenda. Referencing Figure 7, the MOH has made strategic investments to support getting to:
State-A: Well-formed CCGs are available in a Guideline Repository; and
State-B: the updated CCGs exist on a Guideline Engine.
By making investments in shared national infrastructure, Amalgaland has put necessary puzzle pieces in place for the phasing in of its VBHS-centric payments scheme and to leverage digital health to systemically improve the quality of health services across its national care delivery network.
The fictitious organization, Integrated Health (IH), is a large healthcare network serving over a million patients. It has 65 thousand healthcare employees and operates over 30 hospitals and almost 400 clinics. IH is a well-regarded not-for-profit organization that has received national accolades for consistently providing high-quality care in a cost-effective way. To support continuous quality improvement, IH plans to leverage CCGs as a foundational element in its Learning Health System19 (LHS) strategy.

As an integrated provider, IH will operationalize and govern all four of the key use cases IHE CCG Profile:
Digital health teams at its academic health centres have formed a collaborative group that will develop and publish IH’s existing care protocols as FHIR-based CCGs.
To support the LHS strategy, at each encounter, IH’s CCGs will explicitly and deliberately capture patient function using WHO’s International Classification of Functioning, Disability and Health20 (ICF) codes.
To ensure it remains eligible for federal quality payment programs, the IH team will continuously augment its existing CCGs and develop new CCGs as additional federal incentive programs emerge.
All IH’s CCGs (and all its health databases) are based on the national core FHIR data model.
As a long-time leader in digital health, IH is leveraging its deep health informatics expertise to customize a CCG Engine to capture copious runtime information that can be leveraged to support research-based analytics. This CCG Engine will support the entire IH care network and be regularly refreshed with IH’s most up-to-date CCGs.
IH’s strong analytics capacity will be leveraged to help identify patients that should be, but are not now, enrolled in CCG-supported care pathways. The results of these analyses will be made available to both providers and patients during the appointment scheduling workflow.
All IH care sites leverage an EMR platform that connects to the network’s shared health record through a dedicated HIE. This EMR platform will be enhanced to operationalize CCG-supported workflows, including a simplified UI for capturing ICF codes.
IH’s CCG-supported approach will leverage the naturally occurring variation across its large care network. At the same time the broad adoption of CCGs will be ensuring consistent high-quality care delivery, the deliberate systemic capturing of ICF codes will help IH to identify “positive outliers”. In cases where CCGs’ recommendations are not being followed – but where the outcomes are systemically better – IH’s analyses will enable it to continuously improve the CCGs themselves.
Fictitious rural physician, Dr. Black, has plans to purchase a new EMR for her family medicine practice. A key reason for Dr. Black wanting to switch out her existing EMR is the significant amount of time and effort she finds herself expending on “busy work” and seemingly endless data entry. This is time she would rather spend with her patients, or with her young family (she finds herself, now, doing a lot of catch-up data entry outside of clinic hours). The relentlessness of the problem is putting her in danger of physician burnout. Dr. Black has learned about CCGs and believes in their potential to reduce her cognitive load while simultaneously reducing her data entry burden.
The Provincial MOH has published a set of CCGs targeted at particularly prevalent health concerns. An incentive payment program has been put in place to encourage adoption of these new CCGs with additional bonuses for multi-chronic-condition patients. Dr. Black has many such patients in her practice and she has determined that the first year of incentives will fund her plans to implement a new EMR.
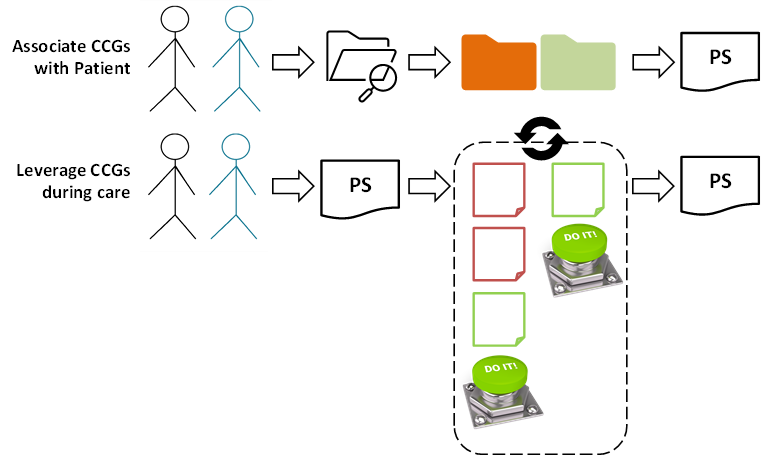
Dr. Black appreciates there will be two complementary aspects to employing CCGs in her practice:
For her patients, including Mr. Blue (for example), she will need to leverage her EMR to lookup the available CCGs that may be applicable. Where she and Mr. Blue agree, she will associate relevant CCGs with his health record and these CCG associations will become part of Mr. Blue’s patient summary.
Thereafter, at each care encounter with Mr. Blue, his patient summary (and the associated CCGs) will be retrieved and leveraged. Over the course of the care encounter, Dr. Black’s EMR will iteratively propose CCG-informed recommendations. If they are non-controversial, these recommendations can be automatically accepted and actioned using the EMR’s “DO IT” button. (If she and Mr. Blue choose to not follow a recommendation, she just indicates the reason and then pushes the button). At the conclusion of the encounter, all the CCG-informed content will automatically be included in Mr. Blue’s updated patient summary.
Dr. Black anticipates that substantially reducing the data entry “busy work” will leave more time for the doctor-patient engagement that motivated her to enter medicine in the first place. Importantly, she knows from the evidence that more consistently adhering to guideline-based care will improve health outcomes for Mr. Blue and for all her patients.
Footnotes
The four levels of knowledge representation are described in the CPG-on-FHIR IG: https://hl7.org/fhir/uv/cpg/documentation-approach-02-04-knowledge-representation.html. A CCG is a level-3 (L3) artifact. ↩ ↩2
https://build.fhir.org/ig/HL7/cqf-recommendations/index.html ↩
Organization of Economic Development country members: https://www.oecd.org/en/about/members-partners.html ↩
Low- and Middle-income Countries as defined by World Bank income stratification: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups ↩
https://www.canada.ca/en/services/health/publications/diseases-conditions/prevalence-chronic-disease-risk-factors-canadians-aged-65-years-older.html ↩
The estimate, from 2017, is that an economic cost of $122 billion (CAD) relates to indirect income and productivity losses, and $68 billion in direct health care costs. ↩
https://www.ourcommons.ca/content/committee/421/fina/brief/br9073636/br-external/chronicdiseasepreventionallianceofcanada-e.pdf ↩
National Academies of Sciences, Engineering, and Medicine. 2018. Crossing the global quality chasm: Improving health care worldwide. Washington, DC: The National Academies Press. (https://www.nap.edu/catalog/25152/crossing-the-global-quality-chasm-improving-health-care-worldwide) ↩
https://www.who.int/news/item/05-07-2018-low-quality-healthcare-is-increasing-the-burden-of-illness-and-health-costs-globally ↩
https://www.healthit.gov/test-method/decision-support-interventions#ccg ↩
https://build.fhir.org/ig/HL7/cqf-recommendations/artifacts.html#activitydefinition-index ↩
The Institute for Health Metrics and Evaluation (IHME) is a trusted research body. Country profiles related to burden of disease are regularly published, along with the supporting data related to disability-adjusted life years (DALYs). https://www.healthdata.org/research-analysis/health-by-location/profiles ↩
https://www.who.int/news/item/23-09-2024-boosting-digital-health-can-help-prevent-millions-of-deaths-from-noncommunicable-diseases ↩
https://iris.who.int/bitstream/handle/10665/340724/9789240020344-eng.pdf?sequence=1 ↩
https://macgrade.mcmaster.ca/resources/gin-mcmaster-guideline-development-checklist/ ↩
https://www.authorea.com/users/701108/articles/687733-gin-mcmaster-guideline-development-checklist-extension-for-computable-guidelines ↩
https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health ↩