De-Identification Profile
0.0.1-current - ci-build
De-Identification Profile
0.0.1-current - ci-build
De-Identification Profile, published by IHE IT Infrastructure Technical Committee. This guide is not an authorized publication; it is the continuous build for version 0.0.1-current built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/IHE/ITI.DeIdHandbook/ and changes regularly. See the Directory of published versions
This exemplar demonstrates how the IHE De-Identification Handbook’s process framework is applied a fictitious epidemiologic example that conforms to the secondary use requirements defined by the EHDS2. This example leverages the International Patient Summary (IPS) and the Vital Records Death Reporting (VRDR) profiles for conveying study data content for this secondary-use scenario. It serves as a concise de-identification example: defining purpose, recipients, multi-stage process, risk thresholds, and element-by-element treatment to preserve research utility while protecting privacy. See the methodology overview in Process.
The permitted purpose for this example is Public Interest research in Public/Occupational Health to study co-morbidities, mortality, occupational risks, and treatment effects. The dataset must be minimized and de-identified to preserve utility (longitudinal trends, outcomes, medication impacts) while controlling re-identification risk per permit conditions.
The region is under threat of outbreak for a novel virus causing influenza-like-symptoms. An approved public health monitoring program wishes to review primary health information from across multiple jurisdictions for impacts of co-morbidities, mortality rates, and occupational health risks. The study also intends to review the population health impact for medication treatments, including vaccination. The purpose of the research request reflects a permitted purpose of use, Public Interest in the area of Public, Occupational Health. Data for this study represents categories from Healthcare, Medicinal products, Areas of Public Health, Areas of Occupational Health, and possibly Serious cross-border threats.
The Health Information Exchange system has defined the following standard permitted uses (Art 53 a-c). The standard healthcare purposes of use specified by ISO TC215 14265: Health Informatics - Classification of purposes for processing personal health information concepts associated with these EHDS2 defined purposes are provided in italicized subbullets:
The Health Information Exchange system has define the following standard are not permitted uses:
No corresponding purpose of use is defined by EHDS2 as either permitted or not permitted, so the assumption is the following ISO-defined standard classifications of use purposes are not permitted uses:
Additional regulation purposes from Article 54 not permitted:
This data flow implements the methodology’s end-to-end analysis described in Process → Analyze the context → Data flow. It maps: source clinical systems → HDAB Intermediation (Stage 2) → controlled recipients, and highlights multi-stage requirements, environments, and constraints.
The following diagram from the EHDS2 M7.2 Draft guideline on data minimisation, pseudonymisation, anonymisation and synthetic data depicts the EHDS user journey for the full process, highlighting the areas within the process that address data minimization, pseudonymization and anonymization:
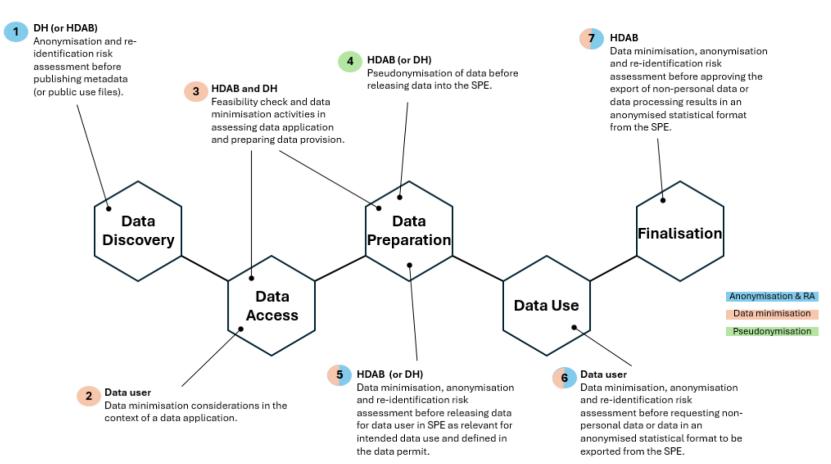
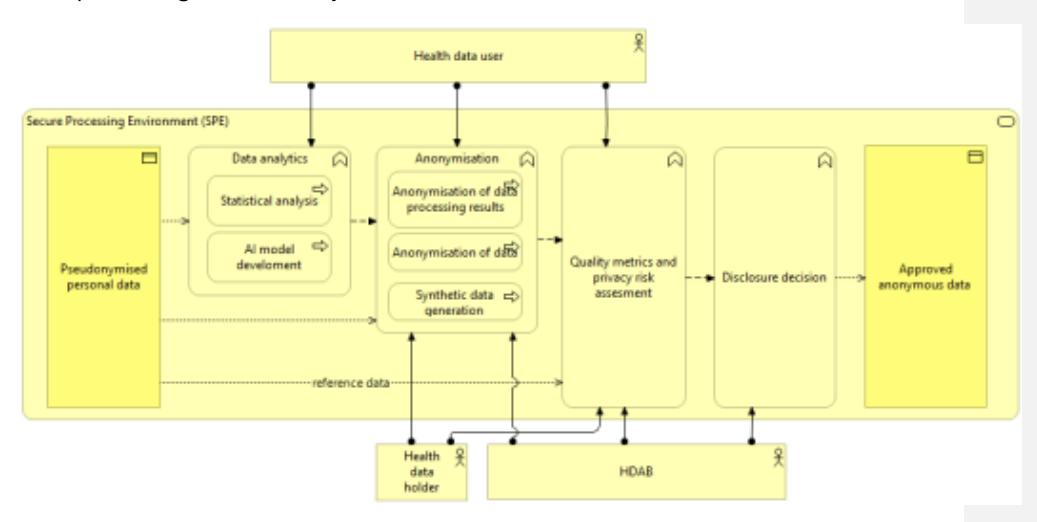
Once a Data Permit is granted, the third phase, Data Preparation, begins. The HDAB prepares the data set according to the permit content and de-identification to be applied. The EHDS2 M7.2 Draft guideline on data minimisation, pseudonymisation, anonymisation and synthetic data depicts a High-level architecture for safe disclosure of anonymized data, processing results, and synthetic data:
The Dataset is primarily structured clinical data from IPS (FHIR R4) and mortality attributes from VRDR (FHIR R4). It is longitudinal (multiple records per subject across time), minimizes free text (procedure descriptions removed), excludes imaging, and contains no binary device logs. Semi-structured risks (filenames, metadata) are minimized by standardized export and HDAB processing.
Policy for secondary use includes data minimisation for the use of secondary health data. This includes limiting the amount, type, and granularity of data during data preparation. The application for data access includes a request for the following data and de-identification methods:
Population comprises patients across multiple jurisdictions potentially affected by an influenza-like outbreak, including general clinical populations, occupational cohorts, and pregnant individuals. Key characteristics: broad age range with age-group generalization; diverse geographies with postal code generalization; mixed gender distribution; inclusion of sensitive clinical conditions (Problems, Immunizations, Mortality) requiring controlled handling.
Distinct types include structured records (IPS sections: Patient, Problems, Procedures, Medications, Allergies, Results, Immunizations, Social/Pregnancy History) and structured mortality data (VRDR). Auxiliary information that can hide identifiers (file names, directory paths, embedded metadata) is excluded or standardized in HDAB pipelines; provenance is retained separately for audit.
Data Types: The IPS format requested contains primarily structured data with some attributes containing textual data content. There are no Medical imaging data, Bio-signal data, Genetic data, Textual data, or Multi-modal data. Available to this research study through the IPS structured format.
This implements the element-by-element de-identification design as defined in Process. The Identifier Type (DI/QI/NI) classification and Handling/Notes capture the selected transformations for each data element available in the source IPS and VRDR standardized content.
| Section | Element | Data Type | Identifier Type | Handling/Notes |
|---|---|---|---|---|
| Patient | Patient Name | Structured | Direct Identifier | Reversibly pseudonymized (name) |
| Patient | ID | Structured | Direct Identifier | Reversibly pseudonymized (identifier) |
| Patient | Date of Birth | Structured | Quasi-identifier | Synthetic data via date shifting within age-group breakdown |
| Patient | Gender | Structured | Quasi-identifier | Included; important study metric |
| Patient | Telecom | Structured | Direct Identifier | Omitted for data minimization |
| Patient | Deceased indicator | Structured | Quasi-identifier | Included |
| Patient | Deceased date | Structured | Quasi-identifier | Synthetic data via date shifting relative to shifted date of birth |
| Patient | Patient address | Structured | Quasi-identifier | Generalize to first 3 digits of postal code |
| Patient | Preferred language | Structured | Quasi-identifier | Not requested; omitted for data minimization |
| Patient | General Practitioner | Structured | Quasi-identifier | Omitted for data minimization |
| Patient | Insurance | Structured | Quasi-identifier | Omitted for data minimization |
| Problems | Problem Type | Structured | Non-identifying | Unchanged |
| Problems | Diagnosis | Structured | Quasi-identifier | Reviewed for potential identifiable outliers for suppression |
| Problems | Severity | Structured | Non-identifying | Unchanged |
| Problems | Onset Date | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Problems | Problem Status | Structured | Non-identifying | Omitted for data minimization |
| Problems | Specialist Contact | Structured | Quasi-identifier | Omitted for data minimization |
| Procedures | Procedure code | Structured | Non-identifying | Unchanged |
| Procedures | Procedure description | Textual | Quasi-identifier | Omitted due to potential free-text privacy issues |
| Procedures | Body site | Structured | Non-identifying | Unchanged |
| Procedures | Procedure date | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Medication Summary | Product code | Structured | Non-identifying | Unchanged |
| Medication Summary | Product common name and strength | Textual | Non-identifying | Unchanged if known; coded product code not required |
| Medication Summary | Active ingredient substance code | Structured | Non-identifying | Unchanged |
| Medication Summary | Active ingredient strength | Structured | Non-identifying | Unchanged |
| Medication Summary | Period of medication use | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Medication Summary | Route of administration | Structured | Non-identifying | Not requested; omitted for data minimization |
| Medication Summary | Dose quantity | Structured | Non-identifying | Unchanged |
| Medication Summary | Dose frequency | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Clinical status | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Onset date | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Allergies/Intolerances | End date | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Allergies/Intolerances | Criticality | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Certainty | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Type of propensity | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Diagnosis | Structured | Quasi-identifier | Omitted for data minimization (outlier risk) |
| Allergies/Intolerances | Reaction manifestation | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Reaction severity | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Agent code | Structured | Non-identifying | Unchanged |
| Allergies/Intolerances | Agent category | Structured | Non-identifying | Unchanged |
| Results | Date of observation | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Results | Observation type | Structured | Non-identifying | Unchanged |
| Results | Result description | Textual | Non-identifying | Not requested; omitted for data minimization |
| Results | Result value | Structured | Non-identifying | Unchanged |
| Results | Observation result | Structured | Non-identifying | Unchanged |
| Results | Performer | Structured | Non-identifying | Omitted for data minimization |
| Results | Observer | Structured | Non-identifying | Omitted for data minimization |
| Immunizations | Vaccine (type of disease) | Structured | Non-identifying | Unchanged |
| Immunizations | Date of immunization | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Immunizations | Number in series of doses | Structured | Non-identifying | Unchanged |
| Immunizations | Target disease | Structured | Non-identifying | Omitted for data minimization |
| Immunizations | Product name | Textual | Non-identifying | Omitted for data minimization |
| Immunizations | Product administration | Structured | Non-identifying | Omitted for data minimization |
| Immunizations | Performer | Structured | Non-identifying | Omitted for data minimization |
| Immunizations | Route of administration | Structured | Non-identifying | Omitted for data minimization |
| Social History | Occupation | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date; review for identifiable outliers |
| Social History | Industry | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date; review for identifiable outliers |
| Pregnancy History | Pregnancy status | Structured | Non-identifying | Unchanged |
| Pregnancy History | Estimated delivery date | Structured | Quasi-identifier | Subject to date shifting relative to shifted birth date |
| Medical Devices | Device data required | Structured | Non-identifying | Not needed for the study; data absent reason marked as 'masked' |
| Mortality | Name | Structured | Direct Identifier | Reversibly pseudonymized (name) |
| Mortality | Identifier | Structured | Direct Identifier | Reversibly pseudonymized (identifier) |
| Mortality | Date of death | Structured | Quasi-identifier | Synthetic data via date shifting within age-group breakdown |
| Mortality | Cause of death | Structured | Quasi-identifier | Reviewed for potential identifiable outliers for suppression |
Direct identifiers (DI) in IPS/VRDR are removed or pseudonymized prior to release:
Patient.name, Patient.telecom, and local Patient.identifier are pseudonymized (reversible at source if operationally required; irreversible within HDAB Intermediation for release). Patient.address is not de-identified in Stage 1, and is generalized (e.g., to 3-digit postal code) in Stage 2.VRDR.name and VRDR.identifier are pseudonymized; VRDR.dateOfDeath is date-shifted within age-band policy. Decedent address (Patient.address in the VRDR document) is generalized in Stage 2.Procedure.description, Observation.note) are omitted to avoid latent identifiers.Quasi-identifiers (QI) intentionally retained for utility include: age group (derived from Patient.birthDate), 3-digit postal code (from Patient.address), Patient.gender, occupation and industry (Social History), clinically relevant event dates (onset, procedure, immunization, observation) after controlled date shifting, pregnancy status, and cause-of-death categories. Sensitive attributes (SA) such as diagnoses, result values, medications are preserved but protected by linkage controls and outlier suppression.
Given remaining QIs, qualitative classification alone is insufficient. After transformations, the target qualitative state is Irreversibly Pseudonymized Data suitable for non-public controlled sharing, pending quantitative risk confirmation.
Apply k-anonymity to the structured IPS/VRDR release using project-specific QIs:
Patient.birthDate → age groups)Patient.address.postalCode)Patient.gender)Condition.onsetDateTime, Procedure.performed[x], Immunization.occurrence[x], Observation.effective[x])Compute equivalence classes on the tuple (AgeBand, Postal3, Gender, OccupationCategory, IndustryCategory, EventDateBuckets). For VRDR, use (AgeBand, Postal3, Gender, DeathMonthBucket, CauseCategory) with cause-of-death aggregated to broad categories to avoid singling out.
Compare overall risk to threshold: enforce average risk ≤ 0.075 while controlling outliers by generalizing buckets (e.g., widen age bands, aggregate occupations/industries, coarsen date buckets) or suppressing small classes. As an additional safeguard, cap maximum risk by ensuring minimum class size (e.g., f ≥ 20) for released groups to keep θ ≤ 0.05 where required for sensitive subsets.
For aggregate publication, apply Differential Privacy to counts/rates using a strict ε consistent with context risk; document privacy budgets and composition across queries.
Use per-record risk θᵢ = 1/fᵢ with class size fᵢ; compute maximum risk R_{d,b} and average risk R_{d,c} across classes; select R_{d,c} for non-public sharing and control high-risk outliers.
Estimate R_c = max(T1 deliberate, T2 inadvertent, T3 breach) per Process. For permit-based controlled sharing, R_c < 1 due to contractual and technical controls; document the rationale and safeguards.
Compute R = R_d × R_c for k-anonymity-based releases and confirm R meets the threshold; for DP aggregates, select ε aligned to context risk and enforce privacy budgets consistently.
Controlled Public Sharing (Data Use Agreement) governed by HDAB permits; enclave access may be used for higher sensitivity analyses.
Anticipate identity, membership, and attribute attacks with background knowledge; mitigate via generalization, date shifting, and outlier suppression.
Primary: k-anonymity for structured IPS releases; Optional: Differential Privacy for published aggregate statistics.
Stage 1 (Preliminary, source/early pipeline): remove obvious direct identifiers; apply reversible pseudonyms where operationally required; prepare generalization plans (e.g., 3-digit postal code).
Stage 1 Element-level de-identification rules
| Section | Data Element | De-id Method |
|---|---|---|
| Patient | Patient Name | Reversibly pseudonymized (name) |
| Patient | ID | Reversibly pseudonymized (identifier) |
| Patient | Telecom | Omitted for data minimization |
| Mortality | Name | Reversibly pseudonymized (name) |
| Mortality | Identifier | Reversibly pseudonymized (identifier) |
Stage 2 (Advanced, HDAB Intermediation): irreversible pseudonymization; date shifting relative to birth/incident; generalization/suppression of quasi-identifiers; outlier review; quantitative risk check (equivalence classes); ensure semantic validity.
Stage 2 Element-level de-identification rules
| Section | Data Element | De-id Method |
|---|---|---|
| Patient | Date of Birth | Date shifting within age-group; suppress rare age combinations |
| Patient | Gender | Included (QI) for stratification; monitor small cells |
| Patient | Deceased | Included (QI) for stratification |
| Patient | Deceased date | Date shifting relative to birth; suppress outliers |
| Patient | Address (postal code) | Generalize to 3-digit prefix; suppress unique areas |
| Patient | Preferred language | Omitted for data minimization |
| Patient | General Practitioner | Omitted for data minimization |
| Patient | Insurance | Omitted for data minimization |
| Problems | Onset date | Date shifting relative to diagnosis |
| Problems | Problem Status | Omitted for data minimization |
| Problems | Specialist Contact | Omitted for data minimization |
| Procedures | Procedure date | Date shifting relative to incident |
| Procedures | Body site | Included (QI) at coded granularity; suppress rare sites |
| Procedures | Procedure description | Omitted due to potential free-text privacy issues |
| Medication Summary | Period of medication use | Date shifting relative to incident |
| Medication Summary | Route of administration | Omitted for data minimization |
| Allergies/Intolerances | Onset date | Date shifting relative to incident |
| Allergies/Intolerances | End date | Date shifting relative to incident |
| Allergies/Intolerances | Diagnosis | Omitted for data minimization (outlier risk) |
| Results | Date of observation | Date shifting relative to incident |
| Results | Result description | Omitted for data minimization |
| Results | Performer | Omitted for data minimization |
| Results | Observer | Omitted for data minimization |
| Immunizations | Date of immunization | Date shifting relative to incident |
| Immunizations | Target disease | Omitted for data minimization |
| Immunizations | Product name | Omitted for data minimization |
| Immunizations | Product administration | Omitted for data minimization |
| Immunizations | Performer | Omitted for data minimization |
| Immunizations | Route of administration | Omitted for data minimization |
| Medical Devices | Device data required | Not needed for the study; data absent reason marked as 'masked' |
| Social History | Occupation & industry | Date shifting for employment dates; generalize industry categories |
| Pregnancy | Estimated date of delivery | Date shifting relative to birth |
| Mortality | Date of death | Date shifting relative to birth; suppress outliers |
| Mortality | Cause of death | Outlier review; suppress highly unique causes |
Stage 3 (Recipient Verification): recipient-side verification of risk level and constraints; use enclave or controlled environments when appropriate.
See Process → Multi-stage design.
Batch IDs, transformation logs, and audit trails generated during de-identification are maintained and protected. Mapping tables and seeds are segregated with strict access controls.
Released dataset classification: Irreversibly Pseudonymized Data for non-public controlled sharing, meeting average risk ≤ 0.075 with safeguards. Residual risks are managed via suppression/generalization and contractual controls; utility confirmed for population metrics and longitudinal trends.
The table maps IPS data elements to their FHIR paths and summarizes the applied de-identification method across stages (Stage 1 for direct identifiers; Stage 2 for quasi-identifiers and minimization). Where elements are removed, Data Absent Reason (masked) is used per FHIR guidance.
| Section | Data Element | FHIR Path | De-id Method |
|---|---|---|---|
| Patient | Patient Name | Patient.name | Stage 1: pseudonymize; retain pseudonym |
| Patient | ID | Patient.identifier | Stage 1: pseudonymize; retain pseudonym |
| Patient | Telecom | Patient.telecom.extension(data-absent-reason) | Stage 1: omit value + DAR masked |
| Patient | Date of Birth | Patient.birthDate | Stage 2: date shift (age-group policy) |
| Patient | Gender | Patient.gender | Included (QI) |
| Patient | Deceased indicator | Patient.deceasedBoolean | Included |
| Patient | Deceased date | Patient.deceasedDateTime | Stage 2: date shift |
| Patient | Address (postal code) | Patient.address[0].postalCode | Stage 2: generalize to 3-digit |
| Patient | Preferred language | Patient.communication.language | Omit (minimization) |
| Patient | General Practitioner | Patient.generalPractitioner.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Patient | Insurance | Patient.extension(url='urn:example:insurance') | Omit (minimization) |
| Problems | Problem Type | Condition.category | Included |
| Problems | Diagnosis | Condition.code | Included; outlier review if needed |
| Problems | Severity | Condition.severity | Included |
| Problems | Onset Date | Condition.onsetDateTime | Stage 2: date shift |
| Problems | Problem Status | Condition.clinicalStatus.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Problems | Specialist Contact | Condition.asserter.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Problems | Verification Status | Condition.verificationStatus | Included |
| Procedures | Procedure code | Procedure.code.coding | Included |
| Procedures | Procedure description | Procedure.code._text.extension(data-absent-reason) | Stage 2: omit text + DAR masked |
| Procedures | Note | Procedure.note.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Procedures | Body site | Procedure.bodySite | Included |
| Procedures | Procedure date | Procedure.performedDateTime | Stage 2: date shift |
| Medication Summary | Product code | MedicationStatement.contained(Medication).code | Included |
| Medication Summary | Common name & strength | Medication.code.text; Medication.ingredient.strength | Include when coded; omit free-text if risky |
| Medication Summary | Active ingredient code | Medication.ingredient.itemCodeableConcept | Included |
| Medication Summary | Period of use | MedicationStatement.effectivePeriod | Stage 2: date shift |
| Medication Summary | Route of administration | MedicationStatement.dosage.route.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Medication Summary | Dose quantity | MedicationStatement.dosage.doseAndRate[0].doseQuantity | Included |
| Medication Summary | Dose frequency | MedicationStatement.dosage.timing | Included |
| Allergies | Clinical status | AllergyIntolerance.clinicalStatus | Included |
| Allergies | Onset date | AllergyIntolerance.onsetDateTime | Stage 2: date shift |
| Allergies | End date | AllergyIntolerance.lastOccurrence | Stage 2: date shift |
| Allergies | Criticality | AllergyIntolerance.criticality | Included |
| Allergies | Certainty | AllergyIntolerance.verificationStatus | Included |
| Allergies | Type of propensity | AllergyIntolerance.type | Included |
| Allergies | Diagnosis (extension) | AllergyIntolerance.extension(url='urn:example:allergy-diagnosis') | Omit (minimization; outlier risk) |
| Allergies | Reaction manifestation | AllergyIntolerance.reaction.manifestation | Included |
| Allergies | Reaction severity | AllergyIntolerance.reaction.severity | Included |
| Allergies | Agent code | AllergyIntolerance.code | Included |
| Allergies | Agent category | AllergyIntolerance.category | Included |
| Results | Date of observation | Observation.effectiveDateTime | Stage 2: date shift |
| Results | Observation type | Observation.code | Included |
| Results | Result value | Observation.valueQuantity | Included |
| Results | Result description | Observation.note.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Results | Performer | Observation.performer.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Results | Observer (extension) | Observation.extension(url='urn:example:observer') | Omit (minimization) |
| Immunizations | Vaccine (type of disease) | Immunization.vaccineCode | Included |
| Immunizations | Date of immunization | Immunization.occurrenceDateTime | Stage 2: date shift |
| Immunizations | Number in series | Immunization.protocolApplied.doseNumberPositiveInt | Included |
| Immunizations | Target disease | Immunization.protocolApplied.targetDisease.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Immunizations | Product name | Immunization.vaccineCode.text | Omit (minimization) |
| Immunizations | Product administration | Immunization.doseQuantity/site/route.extension(data-absent-reason) | Stage 2: omit value(s) + DAR masked |
| Immunizations | Performer | Immunization.performer | Omit (minimization) |
| Social History | Occupation | Observation(valueString) where code=11341-5 | Include; review outliers; date-shift the observation date |
| Social History | Industry | Observation(valueString) where code=21843-6 | Include; review outliers; date-shift the observation date |
| Pregnancy | Pregnancy status | Observation(valueCodeableConcept) where code=82810-3 | Included |
| Pregnancy | Estimated delivery date | Observation(valueDateTime) where code=11778-8 | Stage 2: date shift |
| Medical Devices | Device data required | DeviceUseStatement.device.extension(data-absent-reason) | Stage 2: omit value + DAR masked |
| Medical Devices | Note | DeviceUseStatement.note | Included (non-identifying) |
| Mortality | Name | Observation.extension(url='urn:example:decedentName').valueHumanName | Stage 1/2: pseudonymize |
| Mortality | Identifier | Observation.identifier | Stage 1/2: pseudonymize |
| Mortality | Date of death | Observation.effectiveDateTime | Stage 2: date shift |
| Mortality | Cause of death | Observation.valueCodeableConcept | Included; outlier review |
Example view of the Origional IPS document for a pandemic patient Secondary Use Pandemnic IPS Patient Original Identified IPS Document
{
"resourceType" : "Patient",
"id" : "d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"meta" : {
"profile" : [
🔗 "http://hl7.org/fhir/uv/ips/StructureDefinition/Patient-uv-ips"
]
},
"text" : {
"status" : "generated",
"div" : "<div xmlns=\"http://www.w3.org/1999/xhtml\"><p class=\"res-header-id\"><b>Generated Narrative: Patient d174bd1a-b368-41e6-83a2-af77f2b3c60f</b></p><a name=\"d174bd1a-b368-41e6-83a2-af77f2b3c60f\"> </a><a name=\"hcd174bd1a-b368-41e6-83a2-af77f2b3c60f\"> </a><div style=\"display: inline-block; background-color: #d9e0e7; padding: 6px; margin: 4px; border: 1px solid #8da1b4; border-radius: 5px; line-height: 60%\"><p style=\"margin-bottom: 0px\"/><p style=\"margin-bottom: 0px\">Profile: <a href=\"http://hl7.org/fhir/uv/ips/STU2/StructureDefinition-Patient-uv-ips.html\">Patient (IPS)</a></p></div><p style=\"border: 1px #661aff solid; background-color: #e6e6ff; padding: 10px;\">Patricia Jordana Female, DoB: 1996-05-01 ( urn:oid:1.3.6.1.4.1.21367.2011.2.5.5639#IHEEX-33159)</p><hr/><table class=\"grid\"><tr><td style=\"background-color: #f3f5da\" title=\"Record is active\">Active:</td><td>true</td><td style=\"background-color: #f3f5da\" title=\"Known status of Patient\">Deceased:</td><td colspan=\"3\">2024-06-30</td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Ways to contact the Patient\">Contact Detail</td><td colspan=\"3\">ph: 07 850 9900(Mobile)</td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Patient Links\">Links:</td><td colspan=\"3\"><ul><li>General Practitioner: <a href=\"Bundle-6603561c-2888-4355-9df4-23675f6eb458.html#urn-uuid-9e57d970-d0ae-4a36-908f-1cad06f94f28\">Yaser, Joseph</a></li></ul></td></tr></table></div>"
},
"identifier" : [
{
"system" : "urn:oid:1.3.6.1.4.1.21367.2011.2.5.5639",
"value" : "IHEEX-33159"
}
],
"active" : true,
"name" : [
{
"text" : "Patricia Jordana",
"family" : "Jordana",
"given" : [
"Patricia"
]
}
],
"telecom" : [
{
"system" : "phone",
"value" : "07 850 9900",
"use" : "mobile"
}
],
"gender" : "female",
"birthDate" : "1996-05-01",
"deceasedDateTime" : "2024-06-30",
"generalPractitioner" : [
{
"reference" : "urn:uuid:9e57d970-d0ae-4a36-908f-1cad06f94f28",
"display" : "Yaser, Joseph"
}
]
}{
"resourceType": "Bundle",
"type": "document",
"timestamp": "2024-07-01T00:00:00Z",
"entry": [
{
"fullUrl": "Composition/ips-comp-1",
"resource": {
"resourceType": "Composition",
"id": "ips-comp-1",
"meta": {"profile": ["http://hl7.org/fhir/uv/ips/StructureDefinition/Composition"]},
"status": "final",
"type": {"coding": [{"system": "http://loinc.org", "code": "60591-5", "display": "Patient summary Document"}]},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"date": "2024-07-01T00:00:00Z",
"author": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"title": "International Patient Summary",
"confidentiality": "N",
"section": [
{"title": "Problems", "entry": [{"reference": "Condition/cond-1"}]},
{"title": "Procedures", "entry": [{"reference": "Procedure/proc-1"}]},
{"title": "Medication Summary", "entry": [{"reference": "MedicationStatement/medstmt-1"}]},
{"title": "Allergies and Intolerances", "entry": [{"reference": "AllergyIntolerance/allergy-1"}]},
{"title": "Results", "entry": [{"reference": "Observation/obs-hb-1"}]},
{"title": "Immunizations", "entry": [{"reference": "Immunization/imm-1"}]},
{"title": "Social History", "entry": [{"reference": "Observation/obs-occ-1"}, {"reference": "Observation/obs-ind-1"}]},
{"title": "Pregnancy History", "entry": [{"reference": "Observation/obs-prg-1"}, {"reference": "Observation/obs-edd-1"}]},
{"title": "Medical Devices", "entry": [{"reference": "DeviceUseStatement/dus-1"}]},
{"title": "Mortality", "entry": [{"reference": "Observation/obs-cod-1"}]}
]
}
},
{
"fullUrl": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"resource": {
"resourceType": "Patient",
"id": "d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"identifier": [{"system": "https://standards.digital.health.nz/ns/nhi-id", "value": "ABC1234"}],
"name": [{"family": "JORDANA", "given": ["Patricia"]}],
"telecom": [{"system": "phone", "use": "mobile", "value": "07 850 9900"}],
"gender": "female",
"birthDate": "1956-09-30",
"deceasedBoolean": true,
"deceasedDateTime": "2024-06-30",
"address": [{"postalCode": "3210"}],
"communication": [{"language": {"coding": [{"system": "urn:ietf:bcp:47", "code": "en-NZ"}]}}],
"generalPractitioner": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"extension": [{"url": "urn:example:insurance", "valueString": "NZ-ACC-PLAN"}]
}
},
{
"fullUrl": "Condition/cond-1",
"resource": {
"resourceType": "Condition",
"id": "cond-1",
"category": [{"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-category", "code": "problem-list-item"}]}],
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "59621000", "display": "Essential hypertension"}]},
"severity": {"coding": [{"system": "http://snomed.info/sct", "code": "255604002", "display": "Mild"}]},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"onsetDateTime": "2016-05-25",
"clinicalStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-clinical", "code": "active"}]},
"verificationStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-ver-status", "code": "confirmed"}]},
"asserter": {"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}
}
},
{
"fullUrl": "Procedure/proc-1",
"resource": {
"resourceType": "Procedure",
"id": "proc-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "80146002", "display": "Appendectomy"}], "text": "Laparoscopic appendectomy"},
"note": [{"text": "Laparoscopic appendectomy performed"}],
"bodySite": [{"coding": [{"system": "http://snomed.info/sct", "code": "66754008", "display": "Appendix structure"}]}],
"performedDateTime": "2018-03-10"
}
},
{
"fullUrl": "MedicationStatement/medstmt-1",
"resource": {
"resourceType": "MedicationStatement",
"id": "medstmt-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"contained": [{
"resourceType": "Medication",
"id": "med1",
"code": {"coding": [{"system": "http://www.whocc.no/atc", "code": "J01CA", "display": "Penicillins"}], "text": "Amoxicillin 500 mg"},
"ingredient": [{"itemCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "372687004", "display": "Amoxicillin"}]}, "strength": {"numerator": {"value": 500, "unit": "mg"}, "denominator": {"value": 1, "unit": "tablet"}}}]
}],
"medicationReference": {"reference": "#med1"},
"effectivePeriod": {"start": "2024-01-01", "end": "2024-02-01"},
"dosage": [{
"route": {"coding": [{"system": "http://snomed.info/sct", "code": "26643006", "display": "Oral route"}]},
"doseAndRate": [{"doseQuantity": {"value": 500, "unit": "mg"}}],
"timing": {"repeat": {"frequency": 3, "period": 1, "periodUnit": "d"}}
}]
}
},
{
"fullUrl": "AllergyIntolerance/allergy-1",
"resource": {
"resourceType": "AllergyIntolerance",
"id": "allergy-1",
"patient": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"clinicalStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/allergyintolerance-clinical", "code": "active"}]},
"verificationStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/allergyintolerance-verification", "code": "confirmed"}]},
"type": "allergy",
"category": ["medication"],
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "764146007", "display": "Penicillin"}]},
"extension": [{"url": "urn:example:allergy-diagnosis", "valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "294954003", "display": "Allergy to penicillin"}]}}],
"onsetDateTime": "2015-04-01",
"lastOccurrence": "2015-05-01",
"criticality": "high",
"reaction": [{
"manifestation": [{"coding": [{"system": "http://snomed.info/sct", "code": "271807003", "display": "Rash"}]}],
"severity": "moderate"
}]
}
},
{
"fullUrl": "Observation/obs-hb-1",
"resource": {
"resourceType": "Observation",
"id": "obs-hb-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "718-7", "display": "Hemoglobin"}]},
"effectiveDateTime": "2023-11-01",
"valueQuantity": {"value": 13.2, "unit": "g/dL"},
"interpretation": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/v3-ObservationInterpretation", "code": "N", "display": "Normal"}]},
"note": [{"text": "Routine CBC"}],
"performer": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"extension": [{"url": "urn:example:observer", "valueReference": {"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}}]
}
},
{
"fullUrl": "Immunization/imm-1",
"resource": {
"resourceType": "Immunization",
"id": "imm-1",
"patient": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"vaccineCode": {"coding": [{"system": "http://snomed.info/sct", "code": "1119349007", "display": "COVID-19 vaccine"}], "text": "Comirnaty"},
"occurrenceDateTime": "2024-05-01",
"protocolApplied": [{"doseNumberPositiveInt": 2, "targetDisease": [{"coding": [{"system": "http://snomed.info/sct", "code": "840539006", "display": "COVID-19"}]}]}],
"doseQuantity": {"value": 0.5, "unit": "mL"},
"site": {"text": "Left deltoid region"},
"route": {"coding": [{"system": "http://snomed.info/sct", "code": "34206005", "display": "Intramuscular route"}]},
"performer": [{"actor": {"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}}]
}
},
{
"fullUrl": "Observation/obs-occ-1",
"resource": {
"resourceType": "Observation",
"id": "obs-occ-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "11341-5", "display": "History of Occupation"}]},
"valueString": "Nurse",
"effectiveDateTime": "2020-01-15"
}
},
{
"fullUrl": "Observation/obs-ind-1",
"resource": {
"resourceType": "Observation",
"id": "obs-ind-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "21843-6", "display": "Industry of employment"}]},
"valueString": "Healthcare",
"effectiveDateTime": "2020-01-15"
}
},
{
"fullUrl": "Observation/obs-prg-1",
"resource": {
"resourceType": "Observation",
"id": "obs-prg-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "82810-3", "display": "Pregnancy status"}]},
"valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "77386006", "display": "Pregnant"}]}
}
},
{
"fullUrl": "Observation/obs-edd-1",
"resource": {
"resourceType": "Observation",
"id": "obs-edd-1",
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "11778-8", "display": "Estimated delivery date"}]},
"valueDateTime": "2024-12-01"
}
},
{
"fullUrl": "DeviceUseStatement/dus-1",
"resource": {
"resourceType": "DeviceUseStatement",
"id": "dus-1",
"status": "completed",
"device": {"reference": "Device/eumfh-70-275-2"},
"note": [{"text": "Device data required"}],
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"}
}
},
{
"fullUrl": "Observation/obs-cod-1",
"resource": {
"resourceType": "Observation",
"id": "obs-cod-1",
"identifier": [{"system": "urn:vrdr:id", "value": "VRDR-2024-0001"}],
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "81956-5", "display": "Cause of death"}]},
"effectiveDateTime": "2024-06-30",
"valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "840539006", "display": "COVID-19"}]},
"extension": [{"url": "urn:example:decedentName", "valueHumanName": {"family": "JORDANA", "given": ["Patricia"]}}]
}
}
]
}
Example view of the Pseudonymized Bundle document for the pandemic patient example Secondary Use Pandemnic IPS Patient Pseudonymized IPS Document
{
"resourceType" : "Patient",
"id" : "39c9964c-96b7-442d-afc1-2702106a9e57",
"meta" : {
"profile" : [
🔗 "http://hl7.org/fhir/uv/ips/StructureDefinition/Patient-uv-ips"🔗 ,
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-decedent"
]
},
"text" : {
"status" : "generated",
"div" : "<div xmlns=\"http://www.w3.org/1999/xhtml\"><p class=\"res-header-id\"><b>Generated Narrative: Patient 39c9964c-96b7-442d-afc1-2702106a9e57</b></p><a name=\"39c9964c-96b7-442d-afc1-2702106a9e57\"> </a><a name=\"hc39c9964c-96b7-442d-afc1-2702106a9e57\"> </a><div style=\"display: inline-block; background-color: #d9e0e7; padding: 6px; margin: 4px; border: 1px solid #8da1b4; border-radius: 5px; line-height: 60%\"><p style=\"margin-bottom: 0px\"/><p style=\"margin-bottom: 0px\">Profiles: <a href=\"http://hl7.org/fhir/uv/ips/STU2/StructureDefinition-Patient-uv-ips.html\">Patient (IPS)</a>, <a href=\"http://hl7.org/fhir/us/vrdr/STU3/StructureDefinition-vrdr-decedent.html\">Decedent</a></p></div><p style=\"border: 1px #661aff solid; background-color: #e6e6ff; padding: 10px;\">Jordana Patricia (official) Female, DoB: 1996-08-16 ( Social Beneficiary Identifier: SSN#123456789)</p><hr/><table class=\"grid\"><tr><td style=\"background-color: #f3f5da\" title=\"Known Marital status of Patient\">Marital Status:</td><td colspan=\"3\"><span title=\"Codes:{http://terminology.hl7.org/CodeSystem/v3-MaritalStatus S}\">Never Married</span></td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Ways to contact the Patient\">Contact Detail</td><td colspan=\"3\">5590 Lockwood Drive Danville VA US </td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Nominated Contact: Unknown\">Unknown:</td><td colspan=\"3\"><ul><li>Joe Smith</li></ul></td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Sex on visual inspection at the time of death by the funeral home\"><a href=\"http://hl7.org/fhir/us/vrdr/STU3/StructureDefinition-NVSS-SexAtDeath.html\">NVSS SexAtDeath</a></td><td colspan=\"3\"><span title=\"Codes:{http://hl7.org/fhir/administrative-gender F}\">Female</span></td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Spouse is Alive.\"><a href=\"http://hl7.org/fhir/us/vrdr/STU3/StructureDefinition-SpouseAlive.html\">Spouse Is Alive</a></td><td colspan=\"3\"><span title=\"Codes:{http://terminology.hl7.org/CodeSystem/v2-0136 Y}\">Yes</span></td></tr><tr><td style=\"background-color: #f3f5da\" title=\"The registered place of birth of the patient. A sytem may use the address.text if they don't store the birthPlace address in discrete elements.\"><a href=\"http://hl7.org/fhir/extensions/5.2.0/StructureDefinition-patient-birthPlace.html\">Patient Birth Place</a></td><td colspan=\"3\">Roanoke VA US </td></tr></table></div>"
},
"extension" : [
{
"url" : "http://hl7.org/fhir/us/vrdr/StructureDefinition/SpouseAlive",
"valueCodeableConcept" : {
"coding" : [
{
"system" : "http://terminology.hl7.org/CodeSystem/v2-0136",
"code" : "Y"
}
]
}
},
{
"url" : "http://hl7.org/fhir/us/vrdr/StructureDefinition/NVSS-SexAtDeath",
"valueCodeableConcept" : {
"coding" : [
{
"system" : "http://hl7.org/fhir/administrative-gender",
"code" : "F",
"display" : "Female"
}
]
}
},
{
"url" : "http://hl7.org/fhir/StructureDefinition/patient-birthPlace",
"valueAddress" : {
"city" : "Roanoke",
"state" : "VA",
"country" : "US"
}
}
],
"identifier" : [
{
"type" : {
"coding" : [
{
"system" : "http://terminology.hl7.org/CodeSystem/v2-0203",
"code" : "SB",
"display" : "Social Beneficiary Identifier"
}
]
},
"system" : "http://hl7.org/fhir/sid/us-ssn",
"value" : "123456789"
}
],
"name" : [
{
"use" : "official",
"family" : "Patricia",
"given" : [
"Jordana"
]
}
],
"gender" : "female",
"birthDate" : "1996-08-16",
"address" : [
{
"extension" : [
{
"url" : "http://hl7.org/fhir/us/vr-common-library/StructureDefinition/Extension-within-city-limits-indicator-vr",
"valueCoding" : {
"system" : "http://terminology.hl7.org/CodeSystem/v2-0136",
"code" : "Y",
"display" : "Yes"
}
},
{
"url" : "http://hl7.org/fhir/us/vr-common-library/StructureDefinition/StreetName",
"valueString" : "Lockwood"
}
],
"line" : [
"5590 Lockwood Drive"
],
"city" : "Danville",
"_city" : {
"extension" : [
{
"url" : "http://hl7.org/fhir/us/vr-common-library/StructureDefinition/CityCode",
"valuePositiveInt" : 1234
}
]
},
"district" : "Fairfax",
"_district" : {
"extension" : [
{
"url" : "http://hl7.org/fhir/us/vr-common-library/StructureDefinition/DistrictCode",
"valuePositiveInt" : 321
}
]
},
"state" : "VA",
"country" : "US"
}
],
"maritalStatus" : {
"coding" : [
{
"system" : "http://terminology.hl7.org/CodeSystem/v3-MaritalStatus",
"code" : "S",
"display" : "Never Married"
}
]
},
"contact" : [
{
"relationship" : [
{
"coding" : [
{
"system" : "http://terminology.hl7.org/CodeSystem/v2-0131",
"code" : "U"
}
],
"text" : "Friend of family"
}
],
"name" : {
"text" : "Joe Smith"
}
}
]
}{
"resourceType": "Bundle",
"type": "document",
"timestamp": "2024-07-01T00:00:00Z",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"entry": [
{
"fullUrl": "Composition/ips-comp-1",
"resource": {
"resourceType": "Composition",
"id": "ips-comp-1",
"meta": {
"profile": ["http://hl7.org/fhir/uv/ips/StructureDefinition/Composition"],
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"status": "final",
"type": {"coding": [{"system": "http://loinc.org", "code": "60591-5", "display": "Patient summary Document"}]},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"date": "2024-07-01T00:00:00Z",
"author": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"title": "International Patient Summary",
"confidentiality": "N",
"section": [
{"title": "Problems", "entry": [{"reference": "Condition/cond-1"}]},
{"title": "Procedures", "entry": [{"reference": "Procedure/proc-1"}]},
{"title": "Medication Summary", "entry": [{"reference": "MedicationStatement/medstmt-1"}]},
{"title": "Allergies and Intolerances", "entry": [{"reference": "AllergyIntolerance/allergy-1"}]},
{"title": "Results", "entry": [{"reference": "Observation/obs-hb-1"}]},
{"title": "Immunizations", "entry": [{"reference": "Immunization/imm-1"}]},
{"title": "Social History", "entry": [{"reference": "Observation/obs-occ-1"}, {"reference": "Observation/obs-ind-1"}]},
{"title": "Pregnancy History", "entry": [{"reference": "Observation/obs-prg-1"}, {"reference": "Observation/obs-edd-1"}]},
{"title": "Medical Devices", "entry": [{"reference": "DeviceUseStatement/dus-1"}]},
{"title": "Mortality", "entry": [{"reference": "Observation/obs-cod-1"}]}
]
}
},
{
"fullUrl": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"resource": {
"resourceType": "Patient",
"id": "d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"},
{"system": "http://ihe.net/CodeSystem/deid-handling", "code": "masked", "display": "Contains masked elements"}
]
},
"identifier": [{"system": "urn:example:psyn", "value": "PID-7ac6997e"}],
"name": [{"family": "Psyn", "given": ["001"]}],
"telecom": [{
"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]
}],
"gender": "female",
"birthDate": "1956-09-30",
"deceasedBoolean": true,
"deceasedDateTime": "2024-06-30",
"address": [{"postalCode": "3210"}],
"communication": [{"language": {"coding": [{"system": "urn:ietf:bcp:47", "code": "en-NZ"}]}}],
"generalPractitioner": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"extension": [{"url": "urn:example:insurance", "valueString": "NZ-ACC-PLAN"}]
}
},
{
"fullUrl": "Condition/cond-1",
"resource": {
"resourceType": "Condition",
"id": "cond-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"category": [{"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-category", "code": "problem-list-item"}]}],
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "59621000", "display": "Essential hypertension"}]},
"severity": {"coding": [{"system": "http://snomed.info/sct", "code": "255604002", "display": "Mild"}]},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"onsetDateTime": "2016-05-25",
"clinicalStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-clinical", "code": "active"}]},
"verificationStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-ver-status", "code": "confirmed"}]},
"asserter": {"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}
}
},
{
"fullUrl": "Procedure/proc-1",
"resource": {
"resourceType": "Procedure",
"id": "proc-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "80146002", "display": "Appendectomy"}], "text": "Laparoscopic appendectomy"},
"note": [{"text": "Laparoscopic appendectomy performed"}],
"bodySite": [{"coding": [{"system": "http://snomed.info/sct", "code": "66754008", "display": "Appendix structure"}]}],
"performedDateTime": "2018-03-10"
}
},
{
"fullUrl": "MedicationStatement/medstmt-1",
"resource": {
"resourceType": "MedicationStatement",
"id": "medstmt-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"contained": [{
"resourceType": "Medication",
"id": "med1",
"code": {"coding": [{"system": "http://www.whocc.no/atc", "code": "J01CA", "display": "Penicillins"}], "text": "Amoxicillin 500 mg"},
"ingredient": [{"itemCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "372687004", "display": "Amoxicillin"}]}, "strength": {"numerator": {"value": 500, "unit": "mg"}, "denominator": {"value": 1, "unit": "tablet"}}}]
}],
"medicationReference": {"reference": "#med1"},
"effectivePeriod": {"start": "2024-01-01", "end": "2024-02-01"},
"dosage": [{
"route": {"coding": [{"system": "http://snomed.info/sct", "code": "26643006", "display": "Oral route"}]},
"doseAndRate": [{"doseQuantity": {"value": 500, "unit": "mg"}}],
"timing": {"repeat": {"frequency": 3, "period": 1, "periodUnit": "d"}}
}]
}
},
{
"fullUrl": "AllergyIntolerance/allergy-1",
"resource": {
"resourceType": "AllergyIntolerance",
"id": "allergy-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"patient": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"clinicalStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/allergyintolerance-clinical", "code": "active"}]},
"verificationStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/allergyintolerance-verification", "code": "confirmed"}]},
"type": "allergy",
"category": ["medication"],
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "764146007", "display": "Penicillin"}]},
"extension": [{"url": "urn:example:allergy-diagnosis", "valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "294954003", "display": "Allergy to penicillin"}]}}],
"onsetDateTime": "2015-04-01",
"lastOccurrence": "2015-05-01",
"criticality": "high",
"reaction": [{
"manifestation": [{"coding": [{"system": "http://snomed.info/sct", "code": "271807003", "display": "Rash"}]}],
"severity": "moderate"
}]
}
},
{
"fullUrl": "Observation/obs-hb-1",
"resource": {
"resourceType": "Observation",
"id": "obs-hb-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "718-7", "display": "Hemoglobin"}]},
"effectiveDateTime": "2023-11-01",
"valueQuantity": {"value": 13.2, "unit": "g/dL"},
"interpretation": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/v3-ObservationInterpretation", "code": "N", "display": "Normal"}]},
"note": [{"text": "Routine CBC"}],
"performer": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"extension": [{"url": "urn:example:observer", "valueReference": {"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}}]
}
},
{
"fullUrl": "Immunization/imm-1",
"resource": {
"resourceType": "Immunization",
"id": "imm-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"patient": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"vaccineCode": {"coding": [{"system": "http://snomed.info/sct", "code": "1119349007", "display": "COVID-19 vaccine"}], "text": "Comirnaty"},
"occurrenceDateTime": "2024-05-01",
"protocolApplied": [{"doseNumberPositiveInt": 2, "targetDisease": [{"coding": [{"system": "http://snomed.info/sct", "code": "840539006", "display": "COVID-19"}]}]}],
"doseQuantity": {"value": 0.5, "unit": "mL"},
"site": {"text": "Left deltoid region"},
"route": {"coding": [{"system": "http://snomed.info/sct", "code": "34206005", "display": "Intramuscular route"}]},
"performer": [{"actor": {"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}}]
}
},
{
"fullUrl": "Observation/obs-occ-1",
"resource": {
"resourceType": "Observation",
"id": "obs-occ-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "11341-5", "display": "History of Occupation"}]},
"valueString": "Nurse",
"effectiveDateTime": "2020-01-15"
}
},
{
"fullUrl": "Observation/obs-ind-1",
"resource": {
"resourceType": "Observation",
"id": "obs-ind-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "21843-6", "display": "Industry of employment"}]},
"valueString": "Healthcare",
"effectiveDateTime": "2020-01-15"
}
},
{
"fullUrl": "Observation/obs-prg-1",
"resource": {
"resourceType": "Observation",
"id": "obs-prg-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "82810-3", "display": "Pregnancy status"}]},
"valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "77386006", "display": "Pregnant"}]}
}
},
{
"fullUrl": "Observation/obs-edd-1",
"resource": {
"resourceType": "Observation",
"id": "obs-edd-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "11778-8", "display": "Estimated delivery date"}]},
"valueDateTime": "2024-12-01"
}
},
{
"fullUrl": "DeviceUseStatement/dus-1",
"resource": {
"resourceType": "DeviceUseStatement",
"id": "dus-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"status": "completed",
"device": {"reference": "Device/eumfh-70-275-2"},
"note": [{"text": "Device data required"}],
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"}
}
},
{
"fullUrl": "Observation/obs-cod-1",
"resource": {
"resourceType": "Observation",
"id": "obs-cod-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage1-pseudonymized", "display": "Stage 1 pseudonymized"}
]
},
"identifier": [{"system": "urn:example:psyn", "value": "VRDR-PSYN-0001"}],
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "81956-5", "display": "Cause of death"}]},
"effectiveDateTime": "2024-06-30",
"valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "840539006", "display": "COVID-19"}]},
"extension": [{"url": "urn:example:decedentName", "valueHumanName": {"family": "Psyn", "given": ["001"]}}]
}
}
]
}
Notes:
Example view of the Stage 2 Pseudonymized IPS Bundle document for the pandemic patient example Secondary Use Pandemnic IPS Patient Stage 2 Pseudonymized IPS Document
{
"resourceType" : "Patient",
"id" : "6274d469-7a4d-4a66-a261-e5e7b71af267",
"meta" : {
"profile" : [
🔗 "http://hl7.org/fhir/uv/ips/StructureDefinition/Patient-uv-ips"
]
},
"text" : {
"status" : "generated",
"div" : "<div xmlns=\"http://www.w3.org/1999/xhtml\"><p class=\"res-header-id\"><b>Generated Narrative: Patient 6274d469-7a4d-4a66-a261-e5e7b71af267</b></p><a name=\"6274d469-7a4d-4a66-a261-e5e7b71af267\"> </a><a name=\"hc6274d469-7a4d-4a66-a261-e5e7b71af267\"> </a><div style=\"display: inline-block; background-color: #d9e0e7; padding: 6px; margin: 4px; border: 1px solid #8da1b4; border-radius: 5px; line-height: 60%\"><p style=\"margin-bottom: 0px\"/><p style=\"margin-bottom: 0px\">Profile: <a href=\"http://hl7.org/fhir/uv/ips/STU2/StructureDefinition-Patient-uv-ips.html\">Patient (IPS)</a></p></div><p style=\"border: 1px #661aff solid; background-color: #e6e6ff; padding: 10px;\">PseudoFamily Female, DoB: 1996-08-16 ( urn:oid:1.3.6.1.4.1.21367.2011.2.5.5639#IHEEX-33159)</p><hr/><table class=\"grid\"><tr><td style=\"background-color: #f3f5da\" title=\"Record is active\">Active:</td><td>true</td><td style=\"background-color: #f3f5da\" title=\"Known status of Patient\">Deceased:</td><td colspan=\"3\">2024-10-15</td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Ways to contact the Patient\">Contact Detail</td><td colspan=\"3\"><ul><li>-unknown-</li><li>3210 </li></ul></td></tr><tr><td style=\"background-color: #f3f5da\" title=\"Patient Links\">Links:</td><td colspan=\"3\"><ul><li>General Practitioner: <a href=\"Bundle-6603561c-2888-4355-9df4-23675f6eb458.html#Practitioner_9e57d970-d0ae-4a36-908f-1cad06f94f28\">Practitioner Joseph Yaser</a></li></ul></td></tr></table></div>"
},
"identifier" : [
{
"system" : "urn:oid:1.3.6.1.4.1.21367.2011.2.5.5639",
"value" : "IHEEX-33159"
}
],
"active" : true,
"name" : [
{
"text" : "PseudoFamily",
"family" : "PseudoFamily",
"given" : [
"PseudoGiven"
]
}
],
"telecom" : [
{
"extension" : [
{
"url" : "http://hl7.org/fhir/StructureDefinition/data-absent-reason",
"valueCode" : "masked"
}
]
}
],
"gender" : "female",
"birthDate" : "1996-08-16",
"deceasedDateTime" : "2024-10-15",
"address" : [
{
"postalCode" : "3210"
}
],
"generalPractitioner" : [
{
"reference" : "Practitioner/9e57d970-d0ae-4a36-908f-1cad06f94f28"
}
]
}{
"resourceType": "Bundle",
"type": "document",
"timestamp": "2024-07-01T00:00:00Z",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"entry": [
{
"fullUrl": "Composition/ips-comp-1",
"resource": {
"resourceType": "Composition",
"id": "ips-comp-1",
"meta": {
"profile": ["http://hl7.org/fhir/uv/ips/StructureDefinition/Composition"],
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"status": "final",
"type": {"coding": [{"system": "http://loinc.org", "code": "60591-5", "display": "Patient summary Document"}]},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"date": "2024-07-01T00:00:00Z",
"author": [{"reference": "Practitioner/816cf057-b736-4e08-baed-cc21e081b784"}],
"title": "International Patient Summary",
"confidentiality": "N",
"section": [
{"title": "Problems", "entry": [{"reference": "Condition/cond-1"}]},
{"title": "Procedures", "entry": [{"reference": "Procedure/proc-1"}]},
{"title": "Medication Summary", "entry": [{"reference": "MedicationStatement/medstmt-1"}]},
{"title": "Allergies and Intolerances", "entry": [{"reference": "AllergyIntolerance/allergy-1"}]},
{"title": "Results", "entry": [{"reference": "Observation/obs-hb-1"}]},
{"title": "Immunizations", "entry": [{"reference": "Immunization/imm-1"}]},
{"title": "Social History", "entry": [{"reference": "Observation/obs-occ-1"}, {"reference": "Observation/obs-ind-1"}]},
{"title": "Pregnancy History", "entry": [{"reference": "Observation/obs-prg-1"}, {"reference": "Observation/obs-edd-1"}]},
{"title": "Medical Devices", "entry": [{"reference": "DeviceUseStatement/dus-1"}]},
{"title": "Mortality", "entry": [{"reference": "Observation/obs-cod-1"}]}
]
}
},
{
"fullUrl": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"resource": {
"resourceType": "Patient",
"id": "d174bd1a-b368-41e6-83a2-af77f2b3c60f",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"},
{"system": "http://ihe.net/CodeSystem/deid-handling", "code": "masked", "display": "Contains masked elements"}
]
},
"identifier": [{"system": "urn:example:psyn2", "value": "PID-9f8b1ea1"}],
"name": [{"family": "Psyn", "given": ["001"]}],
"telecom": [{
"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]
}],
"gender": "female",
"birthDate": "1956-10-15",
"deceasedBoolean": true,
"deceasedDateTime": "2024-07-15",
"address": [{"postalCode": "321"}],
"generalPractitioner": [{
"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]
}]
}
},
{
"fullUrl": "Condition/cond-1",
"resource": {
"resourceType": "Condition",
"id": "cond-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"category": [{"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-category", "code": "problem-list-item"}]}],
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "59621000", "display": "Essential hypertension"}]},
"severity": {"coding": [{"system": "http://snomed.info/sct", "code": "255604002", "display": "Mild"}]},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"onsetDateTime": "2016-06-10",
"clinicalStatus": {
"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]
},
"verificationStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/condition-ver-status", "code": "confirmed"}]},
"asserter": {
"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]
}
}
},
{
"fullUrl": "Procedure/proc-1",
"resource": {
"resourceType": "Procedure",
"id": "proc-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {
"coding": [{"system": "http://snomed.info/sct", "code": "80146002", "display": "Appendectomy"}],
"_text": {"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]}
},
"note": [{"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]}],
"bodySite": [{"coding": [{"system": "http://snomed.info/sct", "code": "66754008", "display": "Appendix structure"}]}],
"performedDateTime": "2018-03-25"
}
},
{
"fullUrl": "MedicationStatement/medstmt-1",
"resource": {
"resourceType": "MedicationStatement",
"id": "medstmt-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"contained": [{
"resourceType": "Medication",
"id": "med1",
"code": {"coding": [{"system": "http://www.whocc.no/atc", "code": "J01CA", "display": "Penicillins"}], "text": "Amoxicillin 500 mg"},
"ingredient": [{"itemCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "372687004", "display": "Amoxicillin"}]}, "strength": {"numerator": {"value": 500, "unit": "mg"}, "denominator": {"value": 1, "unit": "tablet"}}}]
}],
"medicationReference": {"reference": "#med1"},
"effectivePeriod": {"start": "2024-01-18", "end": "2024-02-18"},
"dosage": [{
"route": {
"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]
},
"doseAndRate": [{"doseQuantity": {"value": 500, "unit": "mg"}}],
"timing": {"repeat": {"frequency": 3, "period": 1, "periodUnit": "d"}}
}]
}
},
{
"fullUrl": "AllergyIntolerance/allergy-1",
"resource": {
"resourceType": "AllergyIntolerance",
"id": "allergy-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"patient": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"clinicalStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/allergyintolerance-clinical", "code": "active"}]},
"verificationStatus": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/allergyintolerance-verification", "code": "confirmed"}]},
"type": "allergy",
"category": ["medication"],
"code": {"coding": [{"system": "http://snomed.info/sct", "code": "764146007", "display": "Penicillin"}]},
"onsetDateTime": "2015-04-18",
"lastOccurrence": "2015-05-18",
"criticality": "high",
"reaction": [{
"manifestation": [{"coding": [{"system": "http://snomed.info/sct", "code": "271807003", "display": "Rash"}]}],
"severity": "moderate"
}]
}
},
{
"fullUrl": "Observation/obs-hb-1",
"resource": {
"resourceType": "Observation",
"id": "obs-hb-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "718-7", "display": "Hemoglobin"}]},
"effectiveDateTime": "2023-11-18",
"valueQuantity": {"value": 13.2, "unit": "g/dL"},
"interpretation": {"coding": [{"system": "http://terminology.hl7.org/CodeSystem/v3-ObservationInterpretation", "code": "N", "display": "Normal"}]},
"note": [{"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]}],
"performer": [{"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]}]
}
},
{
"fullUrl": "Immunization/imm-1",
"resource": {
"resourceType": "Immunization",
"id": "imm-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"patient": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"vaccineCode": {"coding": [{"system": "http://snomed.info/sct", "code": "1119349007", "display": "COVID-19 vaccine"}]},
"occurrenceDateTime": "2024-05-18",
"protocolApplied": [{
"doseNumberPositiveInt": 2,
"targetDisease": [{"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]}]
}],
"doseQuantity": {"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]},
"site": {"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]},
"route": {"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]}
}
},
{
"fullUrl": "Observation/obs-occ-1",
"resource": {
"resourceType": "Observation",
"id": "obs-occ-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "11341-5", "display": "History of Occupation"}]},
"valueString": "Nurse",
"effectiveDateTime": "2020-01-31"
}
},
{
"fullUrl": "Observation/obs-ind-1",
"resource": {
"resourceType": "Observation",
"id": "obs-ind-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "21843-6", "display": "Industry of employment"}]},
"valueString": "Healthcare",
"effectiveDateTime": "2020-01-31"
}
},
{
"fullUrl": "Observation/obs-prg-1",
"resource": {
"resourceType": "Observation",
"id": "obs-prg-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "82810-3", "display": "Pregnancy status"}]},
"valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "77386006", "display": "Pregnant"}]}
}
},
{
"fullUrl": "Observation/obs-edd-1",
"resource": {
"resourceType": "Observation",
"id": "obs-edd-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "11778-8", "display": "Estimated delivery date"}]},
"valueDateTime": "2024-12-18"
}
},
{
"fullUrl": "DeviceUseStatement/dus-1",
"resource": {
"resourceType": "DeviceUseStatement",
"id": "dus-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"status": "completed",
"device": {"extension": [{"url": "http://hl7.org/fhir/StructureDefinition/data-absent-reason", "valueCode": "masked"}]},
"note": [{"text": "Device data required"}],
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"}
}
},
{
"fullUrl": "Observation/obs-cod-1",
"resource": {
"resourceType": "Observation",
"id": "obs-cod-1",
"meta": {
"security": [
{"system": "http://ihe.net/CodeSystem/deid-status", "code": "stage2-pseudonymized", "display": "Stage 2 pseudonymized"}
]
},
"identifier": [{"system": "urn:example:psyn2", "value": "VRDR-PSYN2-0001"}],
"subject": {"reference": "Patient/d174bd1a-b368-41e6-83a2-af77f2b3c60f"},
"code": {"coding": [{"system": "http://loinc.org", "code": "81956-5", "display": "Cause of death"}]},
"effectiveDateTime": "2024-07-15",
"valueCodeableConcept": {"coding": [{"system": "http://snomed.info/sct", "code": "840539006", "display": "COVID-19"}]},
"extension": [{"url": "urn:example:decedentName", "valueHumanName": {"family": "Psyn", "given": ["001"]}}]
}
}
]
}
Notes:
meta.security tags set to stage2-pseudonymized for Bundle and resources.urn:example:psyn2) while preserving referential integrity.This example shows a minimized VRDR Death Certificate Document Bundle using the VRDR profiles. Only the following data elements are carried forward: Date of Death, Cause of Death, Decedent name, Decedent address, Decedent occupation, and Decedent industry.
| Section | Data Element | FHIR Path | De-id Method |
|---|---|---|---|
| Document | Death Certificate Document | Bundle.meta.profile | VRDR Bundle profile used |
| Document | Death Certificate | Composition.meta.profile | VRDR Composition profile used |
| Decedent | Name | Patient.name | Stage 1: reversible pseudonymization |
| Decedent | Address | Patient.address | Stage 2: generalize |
| Death | Date of death | Observation.valueDateTime where code=81956-5 | Stage 2: date shift (optionally truncate time) |
| Death | Automated underlying cause of death | Observation.valueCodeableConcept where code=80358-5 | Included |
| Social History | Usual occupation | Observation.valueCodeableConcept where code=21843-8 | Included (review outliers) |
| Social History | Usual industry | Observation.component.valueCodeableConcept where component.code=21844-6 | Included (review outliers) |
{
"resourceType": "Bundle",
"type": "document",
"timestamp": "2024-07-01T00:00:00Z",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-certificate-document"
]
},
"entry": [
{
"fullUrl": "Composition/vrdr-death-certificate-1",
"resource": {
"resourceType": "Composition",
"id": "vrdr-death-certificate-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-certificate"
]
},
"status": "final",
"type": {
"coding": [
{
"system": "http://loinc.org",
"code": "64297-5",
"display": "Death certificate"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"date": "2024-07-01T00:00:00Z",
"title": "U.S. Standard Certificate of Death",
"section": [
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "DecedentDemographics"
}
]
},
"entry": [
{"reference": "Patient/decedent-1"},
{"reference": "Observation/vrdr-usual-work-1"}
]
},
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "DeathInvestigation"
}
]
},
"entry": [{"reference": "Observation/vrdr-death-date-1"}]
},
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "CodedContent"
}
]
},
"entry": [{"reference": "Observation/vrdr-auto-ucod-1"}]
}
]
}
},
{
"fullUrl": "Patient/decedent-1",
"resource": {
"resourceType": "Patient",
"id": "decedent-1",
"name": [
{
"family": "MARTIN",
"given": ["Avery"]
}
],
"address": [
{
"line": ["12 Maple Street"],
"city": "Boston",
"state": "MA",
"postalCode": "02110",
"country": "US"
}
]
}
},
{
"fullUrl": "Observation/vrdr-death-date-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-death-date-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-date"
]
},
"status": "final",
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "81956-5",
"display": "Date+time of death"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueDateTime": "2020-04-05T14:30:00Z"
}
},
{
"fullUrl": "Observation/vrdr-usual-work-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-usual-work-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-decedent-usual-work"
]
},
"status": "final",
"category": [
{
"coding": [
{
"system": "http://terminology.hl7.org/CodeSystem/observation-category",
"code": "social-history"
}
]
}
],
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "21843-8",
"display": "History of Usual Occupation"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueCodeableConcept": {
"coding": [
{
"system": "urn:oid:1.3.6.1.4.1.19376.1.5.3.1.3.43.48.3",
"code": "4221",
"display": "Travel agency and related clerks"
}
],
"text": "4221 | Travel agency and related clerks"
},
"component": [
{
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "21844-6",
"display": "History of Usual industry"
}
]
},
"valueCodeableConcept": {
"coding": [
{
"system": "urn:oid:1.3.6.1.4.1.19376.1.5.3.1.3.43.48.2",
"code": "5110",
"display": "Passenger air transport"
}
],
"text": "5110 Passenger air transport"
}
}
]
}
},
{
"fullUrl": "Observation/vrdr-auto-ucod-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-auto-ucod-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-automated-underlying-cause-of-death"
]
},
"status": "final",
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "80358-5",
"display": "Cause of death.underlying [Automated]"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueCodeableConcept": {
"coding": [
{
"system": "http://hl7.org/fhir/sid/icd-10",
"code": "U07.1",
"display": "COVID-19"
}
]
}
}
}
]
}
{
"resourceType": "Bundle",
"type": "document",
"timestamp": "2024-07-01T00:00:00Z",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-certificate-document"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage1-pseudonymized",
"display": "Stage 1 pseudonymized"
}
]
},
"entry": [
{
"fullUrl": "Composition/vrdr-death-certificate-1",
"resource": {
"resourceType": "Composition",
"id": "vrdr-death-certificate-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-certificate"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage1-pseudonymized",
"display": "Stage 1 pseudonymized"
}
]
},
"status": "final",
"type": {
"coding": [
{
"system": "http://loinc.org",
"code": "64297-5",
"display": "Death certificate"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"date": "2024-07-01T00:00:00Z",
"title": "U.S. Standard Certificate of Death",
"section": [
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "DecedentDemographics"
}
]
},
"entry": [
{"reference": "Patient/decedent-1"},
{"reference": "Observation/vrdr-usual-work-1"}
]
},
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "DeathInvestigation"
}
]
},
"entry": [{"reference": "Observation/vrdr-death-date-1"}]
},
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "CodedContent"
}
]
},
"entry": [{"reference": "Observation/vrdr-auto-ucod-1"}]
}
]
}
},
{
"fullUrl": "Patient/decedent-1",
"resource": {
"resourceType": "Patient",
"id": "decedent-1",
"meta": {
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage1-pseudonymized",
"display": "Stage 1 pseudonymized"
}
]
},
"identifier": [
{
"system": "urn:example:psyn",
"value": "VRDR-PID-0001"
}
],
"name": [
{
"family": "Psyn",
"given": ["001"]
}
],
"address": [
{
"line": ["12 Maple Street"],
"city": "Boston",
"state": "MA",
"postalCode": "02110",
"country": "US"
}
]
}
},
{
"fullUrl": "Observation/vrdr-death-date-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-death-date-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-date"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage1-pseudonymized",
"display": "Stage 1 pseudonymized"
}
]
},
"status": "final",
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "81956-5",
"display": "Date+time of death"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueDateTime": "2020-04-05T14:30:00Z"
}
},
{
"fullUrl": "Observation/vrdr-usual-work-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-usual-work-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-decedent-usual-work"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage1-pseudonymized",
"display": "Stage 1 pseudonymized"
}
]
},
"status": "final",
"category": [
{
"coding": [
{
"system": "http://terminology.hl7.org/CodeSystem/observation-category",
"code": "social-history"
}
]
}
],
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "21843-8",
"display": "History of Usual Occupation"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueCodeableConcept": {
"coding": [
{
"system": "urn:oid:1.3.6.1.4.1.19376.1.5.3.1.3.43.48.3",
"code": "4221",
"display": "Travel agency and related clerks"
}
],
"text": "4221 | Travel agency and related clerks"
},
"component": [
{
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "21844-6",
"display": "History of Usual industry"
}
]
},
"valueCodeableConcept": {
"coding": [
{
"system": "urn:oid:1.3.6.1.4.1.19376.1.5.3.1.3.43.48.2",
"code": "5110",
"display": "Passenger air transport"
}
],
"text": "5110 Passenger air transport"
}
}
]
}
},
{
"fullUrl": "Observation/vrdr-auto-ucod-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-auto-ucod-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-automated-underlying-cause-of-death"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage1-pseudonymized",
"display": "Stage 1 pseudonymized"
}
]
},
"status": "final",
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "80358-5",
"display": "Cause of death.underlying [Automated]"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueCodeableConcept": {
"coding": [
{
"system": "http://hl7.org/fhir/sid/icd-10",
"code": "U07.1",
"display": "COVID-19"
}
]
}
}
}
]
}
Notes:
{
"resourceType": "Bundle",
"type": "document",
"timestamp": "2024-07-01T00:00:00Z",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-certificate-document"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage2-pseudonymized",
"display": "Stage 2 pseudonymized"
}
]
},
"entry": [
{
"fullUrl": "Composition/vrdr-death-certificate-1",
"resource": {
"resourceType": "Composition",
"id": "vrdr-death-certificate-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-certificate"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage2-pseudonymized",
"display": "Stage 2 pseudonymized"
}
]
},
"status": "final",
"type": {
"coding": [
{
"system": "http://loinc.org",
"code": "64297-5",
"display": "Death certificate"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"date": "2024-07-01T00:00:00Z",
"title": "U.S. Standard Certificate of Death",
"section": [
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "DecedentDemographics"
}
]
},
"entry": [
{"reference": "Patient/decedent-1"},
{"reference": "Observation/vrdr-usual-work-1"}
]
},
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "DeathInvestigation"
}
]
},
"entry": [{"reference": "Observation/vrdr-death-date-1"}]
},
{
"code": {
"coding": [
{
"system": "http://hl7.org/fhir/us/vrdr/CodeSystem/vrdr-document-section-cs",
"code": "CodedContent"
}
]
},
"entry": [{"reference": "Observation/vrdr-auto-ucod-1"}]
}
]
}
},
{
"fullUrl": "Patient/decedent-1",
"resource": {
"resourceType": "Patient",
"id": "decedent-1",
"meta": {
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage2-pseudonymized",
"display": "Stage 2 pseudonymized"
}
]
},
"identifier": [
{
"system": "urn:example:psyn2",
"value": "VRDR-PID-9f8b1ea1"
}
],
"name": [
{
"family": "Psyn",
"given": ["001"]
}
],
"address": [
{
"state": "MA",
"postalCode": "021",
"country": "US"
}
]
}
},
{
"fullUrl": "Observation/vrdr-death-date-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-death-date-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-death-date"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage2-pseudonymized",
"display": "Stage 2 pseudonymized"
}
]
},
"status": "final",
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "81956-5",
"display": "Date+time of death"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueDateTime": "2020-04-18"
}
},
{
"fullUrl": "Observation/vrdr-usual-work-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-usual-work-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-decedent-usual-work"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage2-pseudonymized",
"display": "Stage 2 pseudonymized"
}
]
},
"status": "final",
"category": [
{
"coding": [
{
"system": "http://terminology.hl7.org/CodeSystem/observation-category",
"code": "social-history"
}
]
}
],
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "21843-8",
"display": "History of Usual Occupation"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueCodeableConcept": {
"coding": [
{
"system": "urn:oid:1.3.6.1.4.1.19376.1.5.3.1.3.43.48.3",
"code": "4221",
"display": "Travel agency and related clerks"
}
],
"text": "4221 | Travel agency and related clerks"
},
"component": [
{
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "21844-6",
"display": "History of Usual industry"
}
]
},
"valueCodeableConcept": {
"coding": [
{
"system": "urn:oid:1.3.6.1.4.1.19376.1.5.3.1.3.43.48.2",
"code": "5110",
"display": "Passenger air transport"
}
],
"text": "5110 Passenger air transport"
}
}
]
}
},
{
"fullUrl": "Observation/vrdr-auto-ucod-1",
"resource": {
"resourceType": "Observation",
"id": "vrdr-auto-ucod-1",
"meta": {
"profile": [
"http://hl7.org/fhir/us/vrdr/StructureDefinition/vrdr-automated-underlying-cause-of-death"
],
"security": [
{
"system": "http://ihe.net/CodeSystem/deid-status",
"code": "stage2-pseudonymized",
"display": "Stage 2 pseudonymized"
}
]
},
"status": "final",
"code": {
"coding": [
{
"system": "http://loinc.org",
"code": "80358-5",
"display": "Cause of death.underlying [Automated]"
}
]
},
"subject": {"reference": "Patient/decedent-1"},
"valueCodeableConcept": {
"coding": [
{
"system": "http://hl7.org/fhir/sid/icd-10",
"code": "U07.1",
"display": "COVID-19"
}
]
}
}
}
]
}
Notes:
This CDA example mirrors the FHIR IPS sample data and uses CDA-specific masking via nullFlavor (e.g., MSK) for removed elements.
The table maps IPS data elements to their CDA paths and summarizes the applied de-identification method across stages (Stage 1 for direct identifiers; Stage 2 for quasi-identifiers and minimization). Where elements are removed, CDA uses nullFlavor = "MSK".
| Section | Data Element | CDA Path | De-id Method |
|---|---|---|---|
| Patient | Patient Name | ClinicalDocument/recordTarget/patientRole/patient/name | Stage 1: mask via nullFlavor='MSK'; Stage 2: remain masked |
| Patient | ID | ClinicalDocument/recordTarget/patientRole/id | Stage 1: pseudonymize; Stage 2: irreversible pseudonym |
| Patient | Telecom | ClinicalDocument/recordTarget/patientRole/telecom | Stage 1/2: omit value; set nullFlavor='MSK' |
| Patient | Date of Birth | ClinicalDocument/recordTarget/patientRole/patient/birthTime | Stage 2: date shift or mask with age band |
| Patient | Gender | ClinicalDocument/recordTarget/patientRole/patient/administrativeGenderCode | Included (QI) |
| Patient | Address (postal code) | ClinicalDocument/recordTarget/patientRole/addr/postalCode | Stage 2: generalize to 3-digit |
| Patient | Preferred language | ClinicalDocument/languageCode | Omit (minimization) |
| Patient | General Practitioner | ClinicalDocument/documentationOf/serviceEvent/performer | Omit (minimization) |
| Patient | Insurance | ClinicalDocument/coverage (local extension) | Omit (minimization) |
| Problems | Diagnosis | ClinicalDocument/component/section[code=10154-3]/entry/observation/code | Included |
| Problems | Onset Date | ClinicalDocument/component/section[code=10154-3]/entry/observation/effectiveTime@value | Stage 2: date shift |
| Problems | Problem Status | ClinicalDocument/component/section[code=10154-3]/entry/observation/statusCode | Omit (minimization) |
| Problems | Specialist Contact | ClinicalDocument/component/section[code=10154-3]/entry/author/assignedAuthor | Omit (minimization) |
| Procedures | Procedure code | ClinicalDocument/component/section[Procedures]/entry/procedure/code | Included |
| Procedures | Procedure description | ClinicalDocument/component/section[Procedures]/entry/procedure/text | Stage 2: omit free-text; nullFlavor='MSK' if present |
| Procedures | Body site | ClinicalDocument/component/section[Procedures]/entry/procedure/targetSiteCode | Included |
| Procedures | Procedure date | ClinicalDocument/component/section[Procedures]/entry/procedure/effectiveTime@value | Stage 2: date shift |
| Medication Summary | Product code | ClinicalDocument/component/section[Medications]/entry/substanceAdministration/consumable/manufacturedProduct/code | Included |
| Medication Summary | Common name & strength | ClinicalDocument/component/section[Medications]/entry/substanceAdministration/consumable/manufacturedProduct/code@displayName | Include when coded; omit free-text if risky |
| Medication Summary | Period of use | ClinicalDocument/component/section[Medications]/entry/substanceAdministration/effectiveTime | Stage 2: date shift |
| Medication Summary | Route of administration | ClinicalDocument/component/section[Medications]/entry/substanceAdministration/routeCode | Stage 2: omit value; nullFlavor='MSK' |
| Medication Summary | Dose quantity | ClinicalDocument/component/section[Medications]/entry/substanceAdministration/doseQuantity | Included |
| Medication Summary | Dose frequency | ClinicalDocument/component/section[Medications]/entry/substanceAdministration/entryRelationship/sequenceNumber | Included (if present) |
| Allergies | Clinical status | ClinicalDocument/component/section[code=48765-2]/entry/observation/statusCode | Included |
| Allergies | Onset date | ClinicalDocument/component/section[code=48765-2]/entry/observation/effectiveTime@value | Stage 2: date shift |
| Allergies | End date | ClinicalDocument/component/section[code=48765-2]/entry/observation/effectiveTime/high@value | Stage 2: date shift |
| Allergies | Reaction manifestation | ClinicalDocument/component/section[code=48765-2]/entry/observation/value | Included |
| Results | Date of observation | ClinicalDocument/component/section[Results]/entry/observation/effectiveTime@value | Stage 2: date shift |
| Results | Observation type | ClinicalDocument/component/section[Results]/entry/observation/code | Included |
| Results | Result value | ClinicalDocument/component/section[Results]/entry/observation/value | Included |
| Results | Result description | ClinicalDocument/component/section[Results]/entry/observation/text | Stage 2: omit; nullFlavor='MSK' |
| Results | Performer | ClinicalDocument/component/section[Results]/entry/observation/performer | Omit (minimization) |
| Results | Observer | ClinicalDocument/component/section[Results]/entry/observation/author/assignedAuthor | Omit (minimization) |
| Immunizations | Vaccine (type of disease) | ClinicalDocument/component/section[code=11369-6]/entry/substanceAdministration/consumable/manufacturedProduct/code | Included |
| Immunizations | Date of immunization | ClinicalDocument/component/section[code=11369-6]/entry/substanceAdministration/effectiveTime@value | Stage 2: date shift |
| Immunizations | Product name | ClinicalDocument/component/section[code=11369-6]/entry/substanceAdministration/consumable/manufacturedProduct/code@displayName | Omit (minimization) |
| Immunizations | Product administration | ClinicalDocument/component/section[code=11369-6]/entry/substanceAdministration/doseQuantity/siteCode/routeCode | Stage 2: omit value(s); nullFlavor='MSK' |
| Immunizations | Performer | ClinicalDocument/component/section[code=11369-6]/entry/substanceAdministration/performer | Omit (minimization) |
| Social History | Occupation | ClinicalDocument/component/section[Social History]/entry/observation[code=11341-5]/value@value | Include; review outliers; date-shift observation date |
| Social History | Industry | ClinicalDocument/component/section[Social History]/entry/observation[code=21843-6]/value@value | Include; review outliers; date-shift observation date |
| Pregnancy | Pregnancy status | ClinicalDocument/component/section[Pregnancy]/entry/observation[code=82810-3]/value@code | Included |
| Pregnancy | Estimated delivery date | ClinicalDocument/component/section[Pregnancy]/entry/observation[code=11778-8]/value@value | Stage 2: date shift |
| Medical Devices | Device data required | ClinicalDocument/component/section[Medical Devices]/entry/supply/participant[@typeCode='DEV'] | Not needed; set nullFlavor='MSK' or omit |
<ClinicalDocument xmlns="urn:hl7-org:v3">
<recordTarget>
<patientRole>
<id root="https://standards.digital.health.nz/ns/nhi-id" extension="ABC1234"/>
<addr><postalCode>3210</postalCode></addr>
<telecom value="tel:+64-07-850-9900" use="HP"/>
<patient>
<name><given>Patricia</given><family>JORDANA</family></name>
<administrativeGenderCode code="F"/>
<birthTime value="19560930"/>
</patient>
</patientRole>
</recordTarget>
<component>
<section>
<code code="10154-3" codeSystem="2.16.840.1.113883.6.1" displayName="Problem List"/>
<entry>
<observation classCode="OBS" moodCode="EVN">
<code code="59621000" codeSystem="2.16.840.1.113883.6.96" displayName="Essential hypertension"/>
<effectiveTime value="20160525"/>
</observation>
</entry>
</section>
</component>
<component>
<section>
<code code="11369-6" codeSystem="2.16.840.1.113883.6.1" displayName="Immunizations"/>
<entry>
<substanceAdministration classCode="SBADM" moodCode="EVN">
<effectiveTime value="20240501"/>
<consumable>
<manufacturedProduct>
<code code="1119349007" codeSystem="2.16.840.1.113883.6.96" displayName="COVID-19 vaccine"/>
</manufacturedProduct>
</consumable>
</substanceAdministration>
</entry>
</section>
</component>
</ClinicalDocument>
Direct identifiers are removed or pseudonymized; removed elements are marked using nullFlavor="MSK".
<ClinicalDocument xmlns="urn:hl7-org:v3">
<recordTarget>
<patientRole>
<id root="urn:example:psyn" extension="PID-7ac6997e"/>
<addr><postalCode>3210</postalCode></addr>
<telecom nullFlavor="MSK"/>
<patient>
<name nullFlavor="MSK"/>
<administrativeGenderCode code="F"/>
<birthTime value="19560930"/>
</patient>
</patientRole>
</recordTarget>
<component>
<section>
<code code="10154-3" codeSystem="2.16.840.1.113883.6.1" displayName="Problem List"/>
<entry>
<observation classCode="OBS" moodCode="EVN">
<code code="59621000" codeSystem="2.16.840.1.113883.6.96" displayName="Essential hypertension"/>
<effectiveTime value="20160525"/>
</observation>
</entry>
</section>
</component>
<component>
<section>
<code code="11369-6" codeSystem="2.16.840.1.113883.6.1" displayName="Immunizations"/>
<entry>
<substanceAdministration classCode="SBADM" moodCode="EVN">
<effectiveTime value="20240501"/>
<consumable>
<manufacturedProduct>
<code code="1119349007" codeSystem="2.16.840.1.113883.6.96" displayName="COVID-19 vaccine"/>
</manufacturedProduct>
</consumable>
</substanceAdministration>
</entry>
</section>
</component>
</ClinicalDocument>
Quasi-identifiers are transformed; removed elements continue to use nullFlavor="MSK". Dates are shifted to match the FHIR Stage 2 example; postalCode generalized to 3 digits.
<ClinicalDocument xmlns="urn:hl7-org:v3">
<recordTarget>
<patientRole>
<id root="urn:example:psyn2" extension="PID-9f8b1ea1"/>
<addr><postalCode>321</postalCode></addr>
<telecom nullFlavor="MSK"/>
<patient>
<name nullFlavor="MSK"/>
<administrativeGenderCode code="F"/>
<birthTime nullFlavor="MSK">
<!-- Age band derived per policy -->
</birthTime>
</patient>
</patientRole>
</recordTarget>
<component>
<section>
<code code="10154-3" codeSystem="2.16.840.1.113883.6.1" displayName="Problem List"/>
<entry>
<observation classCode="OBS" moodCode="EVN">
<code code="59621000" codeSystem="2.16.840.1.113883.6.96" displayName="Essential hypertension"/>
<effectiveTime value="20160610"/>
</observation>
</entry>
</section>
</component>
<component>
<section>
<code code="11369-6" codeSystem="2.16.840.1.113883.6.1" displayName="Immunizations"/>
<entry>
<substanceAdministration classCode="SBADM" moodCode="EVN">
<effectiveTime value="20240518"/>
<consumable>
<manufacturedProduct>
<code code="1119349007" codeSystem="2.16.840.1.113883.6.96" displayName="COVID-19 vaccine"/>
</manufacturedProduct>
</consumable>
</substanceAdministration>
</entry>
</section>
</component>
</ClinicalDocument>
Notes:
nullFlavor (e.g., MSK) to indicate masked/removed content, whereas FHIR uses the Data Absent Reason extension.PID-7ac6997e, PID-9f8b1ea1).Error processing command: Unable to find fragment resource Bundle/5d7d0b42-86be-4630-b284-6a9f3aab05bc pointed to in file /scratch/repo/input/pagecontent/ips-ehds-example