Respiratory Virus Hospitalization Surveillance Network (RESP-NET) Content Implementation Guide, published by HL7 International / Public Health. This guide is not an authorized publication; it is the continuous build for version 2.0.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/fhir-resp-net-ig/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
Respiratory Virus Hospitalization Surveillance Network (RESP-NET) cases are currently identified through a variety of mechanisms at each site, including review of daily or weekly hospital and laboratory line lists and reportable disease databases, through an often manual and labor-intensive process. Additionally, for laboratory-confirmed hospitalized cases identified through RESP-NET, surveillance staff conduct medical chart abstractions based on a standardized case report form to obtain detailed demographic and clinical data on hospitalized cases including length of stay, underlying medical conditions, hospital course, and in-hospital deaths.
CDC seeks to expand the utilization of electronic health information in RESP-NET and improve data exchange between healthcare facilities, state/local public health partners, and CDC to maintain the quality of these important surveillance activities while improving the efficiency and timeliness with which these data are available. Such enhancements will reduce the burden on surveillance sites and allow for scalability to additional sites in the future.
The goals of the RESP-NET reporting use cases include:
The following actors are used by the RESP-NET use cases:
This section outlines the high-level interactions between the actors listed above.
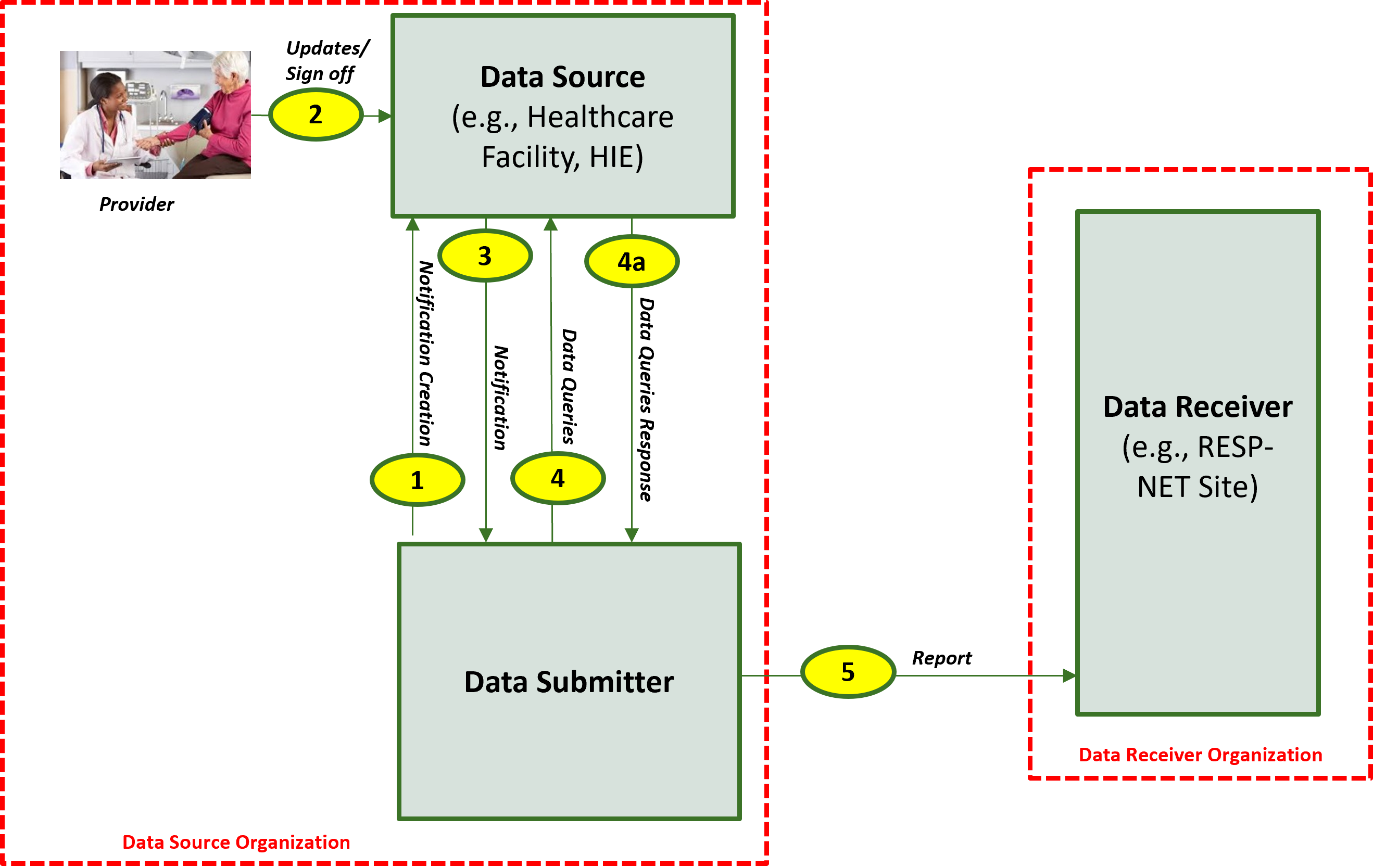
The descriptions for each step in the above diagram where the Data Submitter is hosted within the Data Source Organization are as follow:
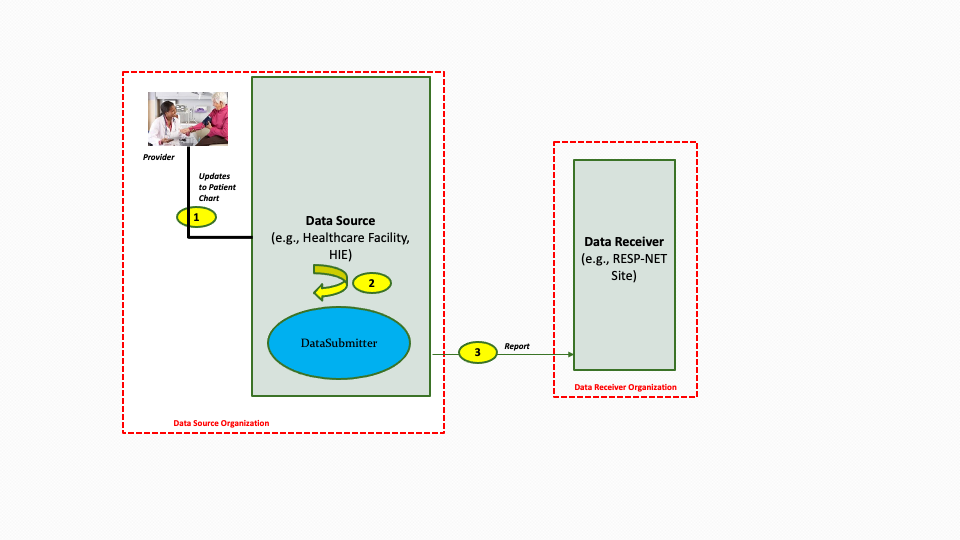
Implementers may package the Data Submitter within the DataSource as illustrated in the diagram below.
The descriptions for each step in the above diagram are as follows:
The next section identifies the list of RESP-Net use cases and provides brief descriptions for the use cases
This use case will identify patients hospitalized with laboratory-confirmed influenza among residents of the RESP-NET catchment area (defined by patient state and county of residence (or other geographic regions)) for active population-based surveillance. The cases are reported along with minimum patient-level data and transmitted to the RESP-NET surveillance officers in the relevant state/local jurisdiction.
Use Case 1 is reported when case identification is met. Minimum data elements to define the catchment area, patient age or date of birth, sex, race/ethnicity, hospital admission date, hospital facility name, positive influenza testing data (test type, test result, test date), and influenza type and subtype (if available).
The positive influenza test prompts a notification to the Data Submitter. The Data Submitter queries for a limited set of PII, including the patient’s address. If the patient is not a resident of a catchment area, the Data Submitter stops all activity regarding this patient. If the patient is a resident of a catchment area, the Data Submitter requests a set of FHIR resources representing patient-level encounter information from the Data Source. The obtained resources are bundled and transmitted to the RESP-NET Site.
Use Case 2 focuses on the more detailed clinical surveillance data collected in RESP-NET by site surveillance officers. Patient-level data from EHRs about persons hospitalized with influenza include data on important outcomes and indicators of disease severity such as ICU admission, mechanical ventilation, in-hospital death, and length of hospital stay. Additional elements include health history (underlying conditions, tobacco, alcohol, substance use), clinical course, viral and bacterial codetections, findings from chest imaging, diagnoses, vaccination, and treatment. This use case will include person-level clinical data elements that are currently collected in RESP-NET on a standardized case report form for all identified cases. Not all of the current data elements may be reachable in a FHIR-based approach; proposed solutions should recognize that this may evolve over time and surveillance officers may still need to conduct medical chart review on data elements.
The close of an encounter prompts a notification to the Data Submitter. The Data Submitter (after a 72-hour delay for lab results) queries for a limited set of data, including the patient’s address, in-patient status, and influenza lab result. If the patient is not a resident of a catchment area, the Data Submitter stops all activity regarding this patient. If the patient is a resident of a catchment area, was admitted to the hospital, and has a positive influenza lab result, the Data Submitter requests a set of FHIR resources representing patient-level encounter information from the Data Source. The obtained resources are bundled and transmitted to the RESP-NET Site.
Because testing for influenza and other respiratory viruses is done at the discretion of the clinician and testing practices may vary widely among facilities and over time, some people hospitalized with influenza may not be recognized and diagnosed. To better estimate the full burden of influenza, CDC collects additional information from RESP-NET hospitals on the proportion of patients with acute respiratory illnesses (ARI) who are tested for influenza, SARS-CoV-2, and RSV. This use case will focus on obtaining data on respiratory viral testing practices for CDC’s RESP-NET disease burden project. This project collects patient-level data on persons hospitalized with ARI (based on ICD-10 diagnosis codes) along with information about whether or not the patient was tested for influenza, SARS-CoV-2, or RSV and if so, the test result and test type used for all tests performed (patients may be tested multiple times). Additional data elements include state, age, race/ethnicity, week of admission, ICD-10 discharge diagnoses, and if possible, whether the patient had been admitted to the ICU or died during the hospital stay.
The close of an encounter prompts a notification to the Data Submitter. The Data Submitter (after a 72-hour delay for lab results) queries for a limited set of PII data, including the patient’s address, in-patient status, and ARI lab result. If the patient is not a resident of a catchment area, the Data Submitter stops all activity regarding this patient. If the patient is a resident of a catchment area, was admitted to the hospital, and has a positive ARI lab result, the Data Submitter requests a set of FHIR resources representing patient-level encounter information from the Data Source. The obtained resources are bundled and transmitted to the RESP-NET site.
These use cases focus on collecting equivalent data to Use Case 2 but for patients who test positive for SARSCoV-2 (the virus that causes COVID-19) (Use Case 4) or Respiratory Syncytial Virus (RSV) (Use Case 5). Public health surveillance through COVID-NET and RSV-NET is conducted in many of the same sites as RESP-NET, collects many of the same data elements, with some additional data elements specific to these viral illnesses.
The close of an encounter prompts a notification to the Data Submitter. The Data Submitter (after a 72-hour delay for lab results) queries for a limited set of data, including the patient’s address, in-patient status, and ARI lab result. If the patient is not a resident of a catchment area, the Data Submitter stops all activity regarding this patient. If the patient is a resident of a catchment area, was admitted to the hospital, and has a positive SARSCoV-2 or RSV lab result, the Data Submitter requests a set of FHIR resources representing patient-level encounter information from the Data Source. The obtained resources are bundled and transmitted to the RESP-NET site.