Consolidated CDA (C-CDA), published by Health Level Seven. This guide is not an authorized publication; it is the continuous build for version 5.0.0-ballot built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/CDA-ccda/ and changes regularly. See the Directory of published versions
The following document provides guidance to readers of the Consolidated CDA Implementation Guide published using HL7 Structure Definition. With the switch to representing C-CDA templates using StructureDefinition, the presentation of the templates is different. Although all the same content is present, it can be found in different places and in different formats. This guide highlights where all template content can be found.
The Artifact Index/Summary page presents the templates that had been previously located in Volume 2. The Artifact Summary page lists the Document Templates, the Section Templates, the Entry Templates, and the Other Templates. As well, there is a listing of all the Examples found in the guide.
C-CDA Templates are represented as Templates in the Implementation Guide. There are several different ways of viewing a Template. The most useful tab is the Key Elements Table, but the Differential Table does provide some information and the Snapshot Table presents all the elements present in the template. The Statistics/References tab shows a summary of elements and a list of templates that are used by the template.

All the Templates inherit from either a CDA Clinical Statement structure or from another Template. The Key Elements Table tab shows all of the changes from the base CDA structure including any changes from a parent Template. The Differential Table shows only the differences between a Template and the parent Template. See the Allergy-Intolerance Observation template for an example of the difference between the two tabs. To see everything that is specified in a template, looking at the Snapshot Table will show all of the elements.
The Template Identifier is listed as another identifier of the template.

The Context tables in the pdf publication that list the templates that contain a given template and the templates that are contained by the same template are represented in two different manners.
The Contained By templates:
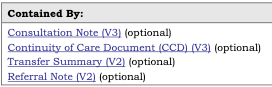
are listed in the Usage section of the Template:

The Contained templates:
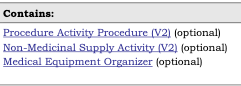
are listed in the Statistics/References tab under the Structures heading:

NOTE: The indication of optional or required for contained by and contains is not present in the corresponding template. This can be seen in Figure 7 where the three templates have “(optional)” while the templates in Figure 8 do not. This means that sometimes a template may be listed twice in the Contained By or Contains in the pdf publication, (once as required and once as optional) but will only be listed once in the corresponding template documentation.
All the template constraints can be found in two equivalent places in the template listing. The Key Elements Table tab presents constraints as they affect the underlying structure (Document, Section, Entry, or Other). Cardinality constraints, fixed value constraints, template constraints, and terminology constraints will all be found in the Differential Table. Some of the text constraints that affect a specific element can also be found in the Key Elements Table.
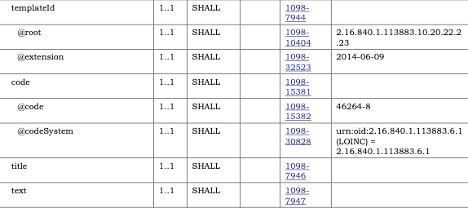
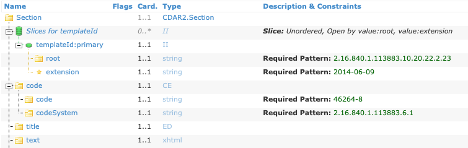
NOTE: For elements that repeat but have been constrained, the Template will be “sliced”, and the constraint will be found on one of the slices. This applies to fixed value constraints as well as template constraints.
NOTE: If a template inherits constraints from a parent template, e.g., Care Team Member Act inherits from Care Team Organizer, the full constraints will not be present in the Differential Tab of the child template but will be found in in the Snapshot Tab of the child template, or in the Differential Tab of the parent template.
The Detailed Descriptions tab will have all the Template Constraints listed by the specific element being constrained. This is where constraint numbers as found in the pdf publication are asserted.

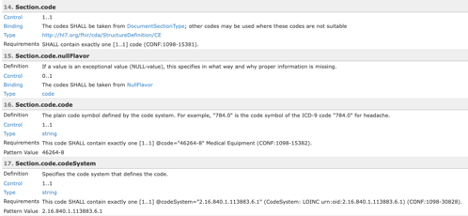
All terminology constraints are represented as value set bindings in the template listing. All bound value sets are represented by the name of the value set and this name is a hyperlink. Many of the value sets can be found in National Library of Medicine’s Value Set Authority Center (VSAC) and will require a UMLS login to see the value set content. SHALL constraints are represented as Required bindings while SHOULD and MAY are represented as Preferred bindings.
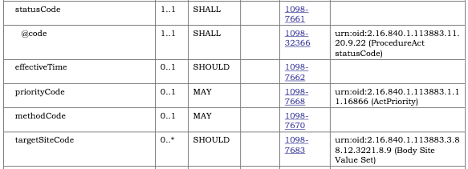

All template constraints that refer to a separate template (e.g., document templates referencing section or entry templates) are represented as specific Types in the Template.
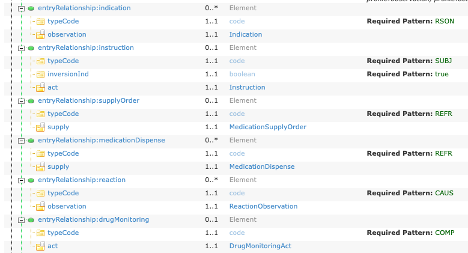
In Figure 13, there are six entryRelationship slices that refer to other templates and this is shown in the Element Type column. The Type name is the Template Name with all spaces and punctuation removed, e.g., Medication Supply Order becomes MedicationSupplyOrder. Each name is a hyperlink to the appropriate template definition.
If a template has examples, those will be found listed on the Examples tab of the template. Each example will be listed with its name which is a hyperlink to the example XML instance.
In previous releases of the C-CDA guide, templates were versioned in two ways. The template templateId would receive a new extension and the name of the template would have "(Vn)" appended to it. With this release of the C-CDA guide, only the templateId extensions will change. The names will remain consistent from one release to another and will no longer have "(Vn)" appended to it. Any templates that did have the Vn in their name have had it removed.
Feedback on the C-CDA Structured Definition web publication can be made by clicking the “Propose a change” at the bottom of every page.

Following that link will take the reader to the HL7 JIRA. On JIRA, clicking on the Create button will allow one to create an issue against the HL7 Software Change Request project. The component should be set to “C-CDA Templates Clinical Notes”. A clear summary and description of the issue should be submitted.