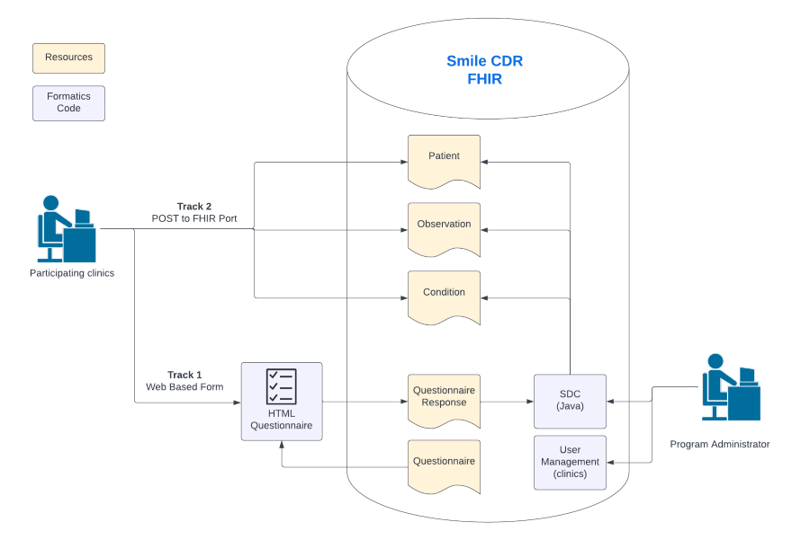
University of the Philippines Manila Viral Hepatitis Project Implementation Guide
0.2.1 - CI Build
University of the Philippines Manila Viral Hepatitis Project Implementation Guide
0.2.1 - CI Build
University of the Philippines Manila Viral Hepatitis Project Implementation Guide, published by UP Manila Viral Hepatitis Project. This guide is not an authorized publication; it is the continuous build for version 0.2.1 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/gpvillanuevasilab/UPVH_WHO_SMART-Template/ and changes regularly. See the Directory of published versions
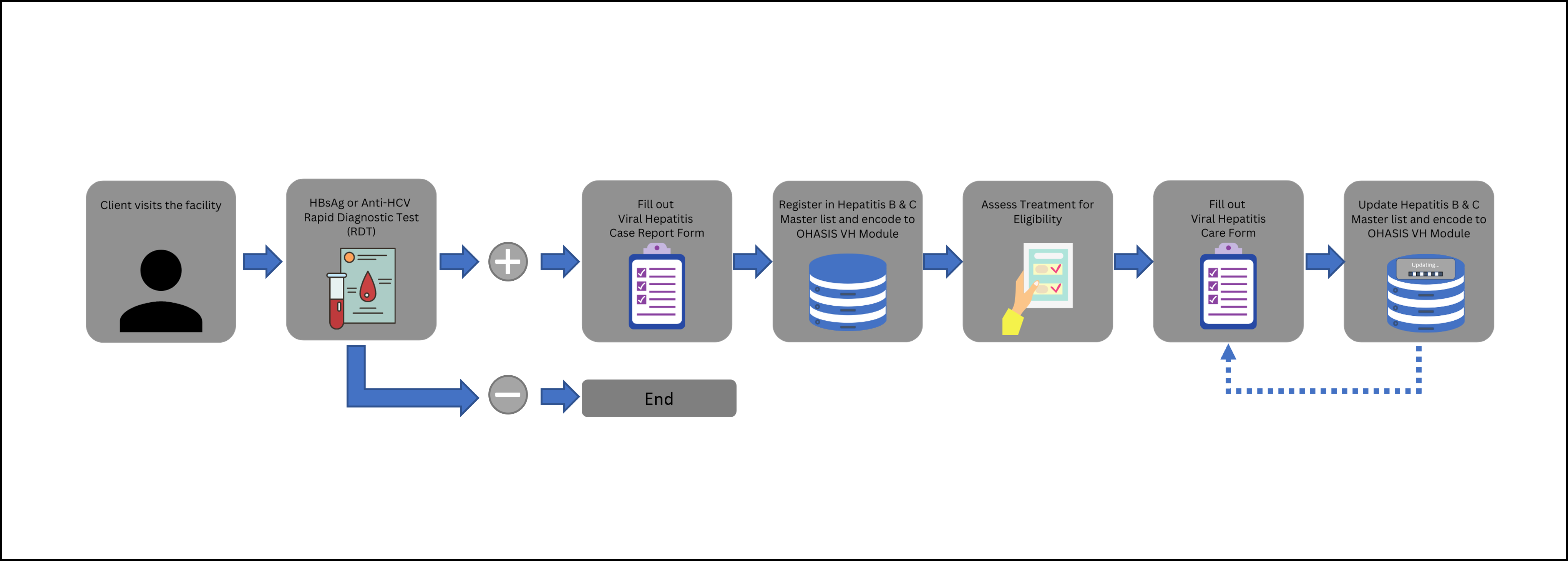
1. Client visits the facility.
2. Screening of client for HBsAg or Anti-HCV tests.
3. Fill out the VIRAL HEPATITIS CASE REPORT FORM (For clients, with reactive HBsAg and Anti-HCV RDT results;).
4. Register client in Hepatitis B and C Masterlist and encode to OHASIS VH Module.
5. Assess for treatment eligibility, and use the VIRAL HEPATITIS CARE FORM.
6. Update the Hepatitis B and C Masterlist and encode to OHASIS VH Module.
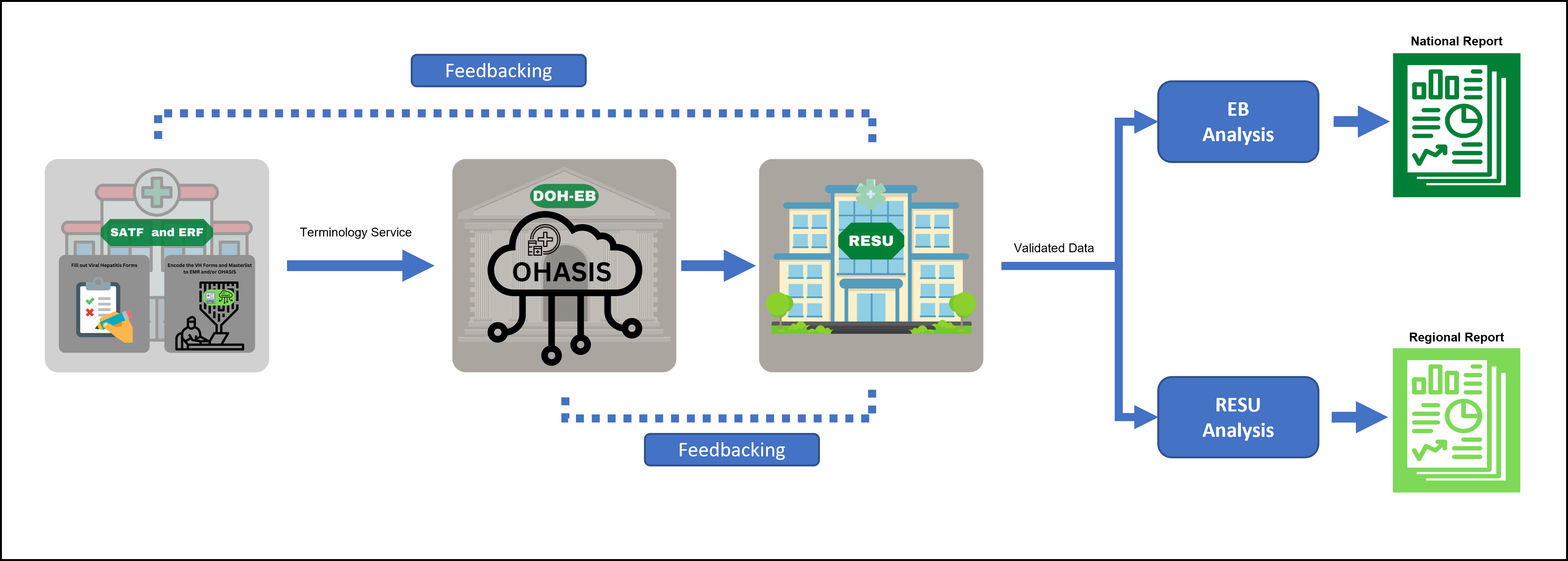
1. SATF and ERF will encode the filled-out Viral Hepatitis forms and masterlist through their own EMR or theough the OHASIS
2. DOH EB will process the data exports from OHASIS and all other EMRs, and will send a viral hepatitis database. Once validated, DOH-EB will send a clean database
3. RESU will disseminate and consolidate the validated data from the facility and revert back to DOH-EB
4. Validated Data will undergo Analysis and to present National Report (DOH-EB) and Regional Report (RESU)