2022 CDC Clinical Practice Guideline for Prescribing Opioids Implementation Guide
2022.1.0 - CI Build
2022 CDC Clinical Practice Guideline for Prescribing Opioids Implementation Guide, published by Centers for Disease Control and Prevention (CDC). This guide is not an authorized publication; it is the continuous build for version 2022.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/opioid-cds-r4/ and changes regularly. See the Directory of published versions
Recommendations #10 (2022 CDC Clinical Practice Guideline for Prescribing Opioids for Pain):
When prescribing opioids for subacute or chronic pain, clinicians should consider the benefits and risks of toxicology testing to assess for prescribed medications as well as other prescribed and nonprescribed controlled substances (recommendation category: B; evidence type: 4).
Note that this recommendation is represented in multiple variations where each variation corresponds to the CDS Hook by which it was meant to be triggered/evaluated. Use the tabs below to navigate to each variation.
Contents
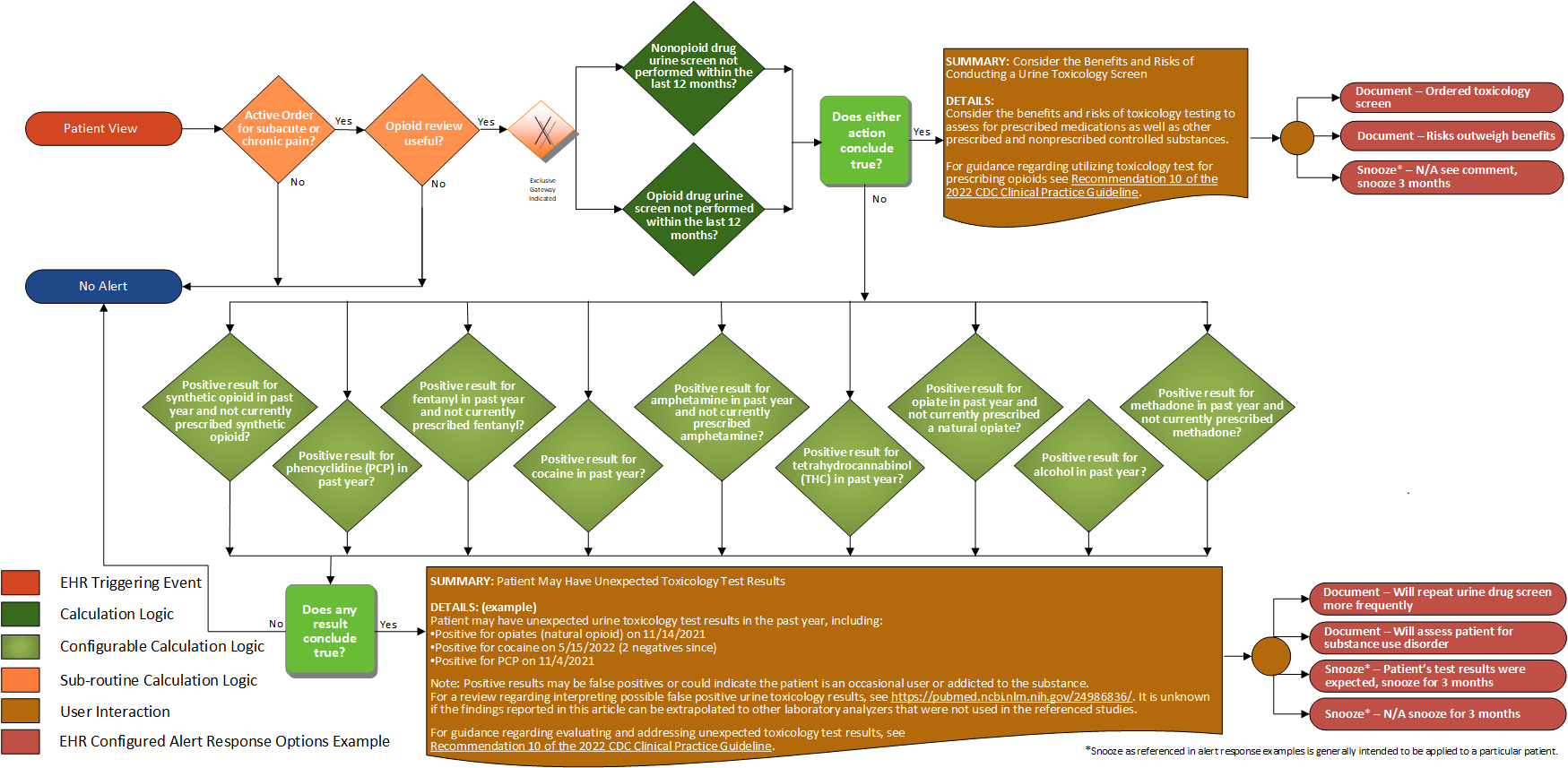
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Opioid order for subacute or chronic pain? | Yes | See For Subacute or Chronic Pain sub-routine | |||
| Opioid review useful? | Yes | See Opioid Review Useful sub-routine | |||
| Non-opioid drug urine screen test not performed within the last 12 months | Yes | No urine screening test performed within the last 12 months | Non-opioid drug urine screen | Observation | Observation.category, Observation.code, Observation.effective, and Observation.status |
| Opioid drug urine screen test not performed within the last 12 months? | Yes | No opioid drug urine screening test performed within the last 12 months | Opioid drug urine screen | Observation | Observation.category, Observation.code, Observation.effective, and Observation.status |
| Urine drug screen results contain cocaine? | Yes | Urine drug screen result contains cocaine | Cocaine urine toxicology test result | Observation | Observation.category, Observation.code, and Observation.value |
| Urine drug screen results contain phencyclidine (PCP)? | Yes | Urine drug screen result contains phencyclidine (PCP) | Phencyclidine (PCP) urine toxicology test result | Observation | Observation.category, Observation.code, and Observation.value |
| Urine drug screen results contain amphetamine and no active amphetamine medication? | Yes | Urine drug screen result contains amphetamine and patient does not have an active prescription for amphetamine | Amphetamine urine toxicology test result Amphetamine medication |
Observation MedicationRequest | Observation.category, Observation.code, Observation.value, MedicationRequest.status, MedicationRequest.authoredOn, and MedicationRequest.medication |
| Urine drug screen results contain opiate and no active opiate medication? | Yes | Urine drug screen result contains opiate and patient does not have an active prescription for opiate | Opiate urine toxicology test result Opiate medication |
Observation MedicationRequest | Observation.category, Observation.code, Observation.value, MedicationRequest.status, MedicationRequest.authoredOn, and MedicationRequest.medication |
| Urine drug screen results contain methadone and no active methadone medication? | Yes | Urine drug screen result contains methadone and patient does not have an active prescription for methadone | Methadone urine toxicology test result Methadone medication |
Observation MedicationRequest | Observation.category, Observation.code, Observation.value, MedicationRequest.status, MedicationRequest.authoredOn, and MedicationRequest.medication |
| Urine drug screen results contain synthetic opioid and no active synthetic opioid medication? | Yes | Urine drug screen result contains synthetic opioid and patient does not have an active prescription for synthetic opioid | Synthetic opioid urine toxicology test result Synthetic opioid medication |
Observation MedicationRequest | Observation.category, Observation.code, Observation.value, MedicationRequest.status, MedicationRequest.authoredOn, and MedicationRequest.medication |
| Urine drug screen results contain fentanyl and no active fentanyl medication? | Yes | Urine drug screen result contains fentanyl and patient does not have an active prescription for fentanyl | Fentanyl urine toxicology test result Fentanyl medication |
Observation MedicationRequest | Observation.category, Observation.code, Observation.value, MedicationRequest.status, MedicationRequest.authoredOn, and MedicationRequest.medication |
| Urine drug screen results contain tetrahydrocannabinol (THC)? | Yes | Urine drug screen result contains tetrahydrocannabinol (THC) | Tetrahydrocannabinol urine toxicology test result | Observation | Observation.category, Observation.code, and Observation.value |
| Urine drug screen results contain alcohol? | Yes | Urine drug screen result contains alcohol | Alcohol urine toxicology test result | Observation | Observation.category, Observation.code, and Observation.value |
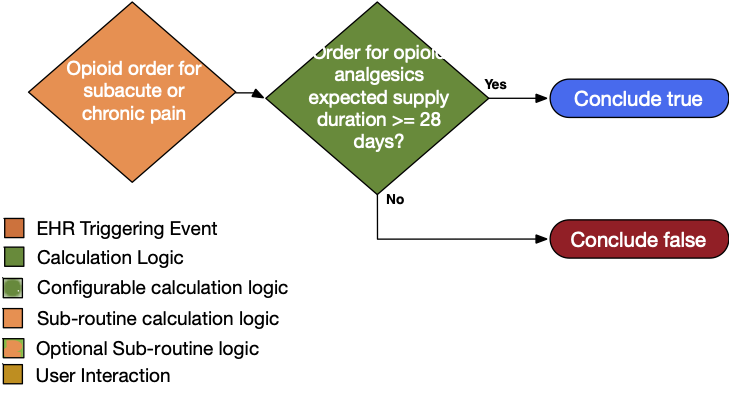
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Order for opioid analgesic with expected supply duration ≥ 28 days | Yes | Order for opioid analgesics with ambulatory misuse potential with a supply duration ≥ 28 days • Subacute definition = order for opioid analgesic with ambulatory misuse potential with a supply duration of one to two months • Chronic pain definition = order for opioid analgesic with ambulatory misuse potential with a supply duration of ≥ two months |
Opioid analgesics with ambulatory misuse potential | MedicationRequest | MedicationRequest.medication and MedicationRequest.dispenseRequest.expectedSupplyDuration |
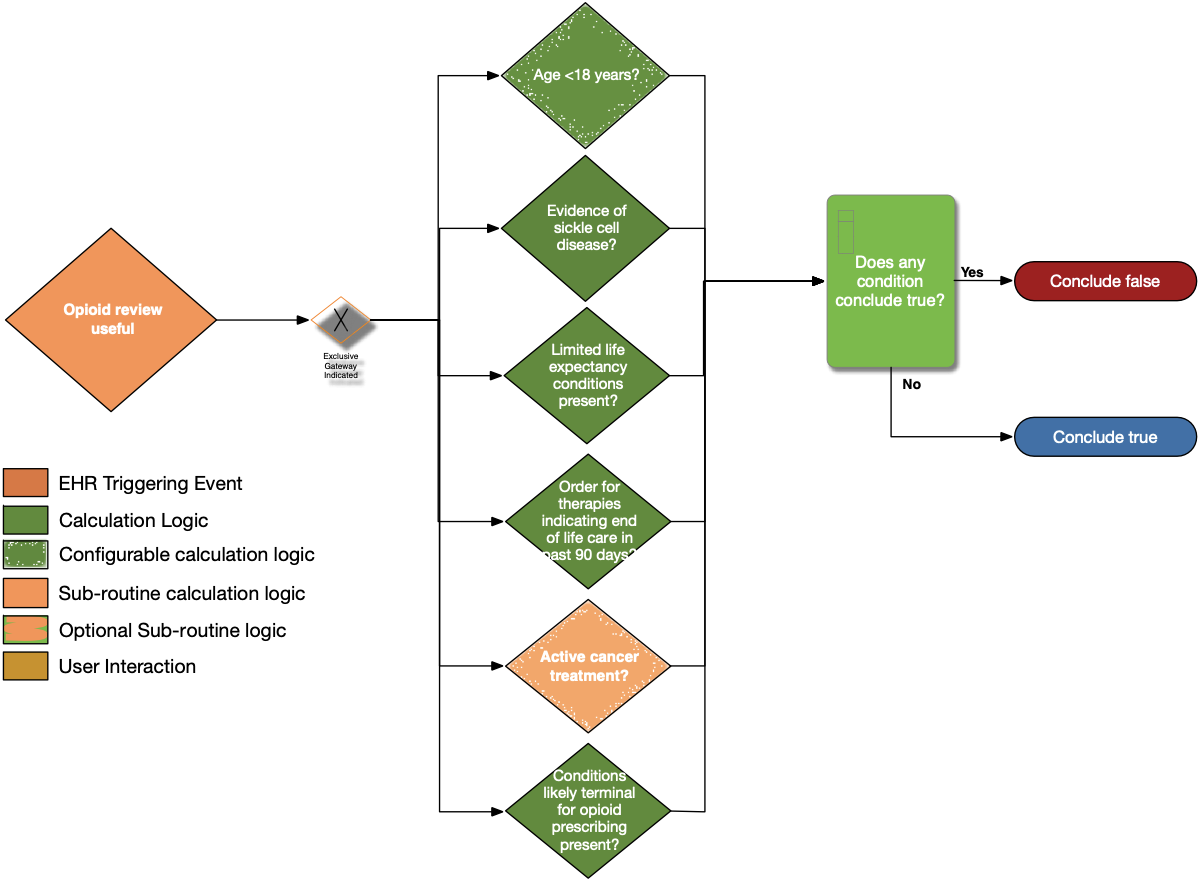
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Age < 18 years? | No | Calculate age from date of birth; exclude patients with age less than 18 years at the time of the prescription | Date of birth | Patient | Patient.birthDate |
| Evidence of sickle cell disease? | No | Look for patients with a diagnosis or problem list entry indicating sickle cell disease | Sickle cell disease condition | Condition | Condition.category, Condition.code, and Condition.clinicalStatus |
| Limited life expectancy conditions present? | No | Look for documented findings consistent with those listed in the limited life expectancy value set (terminal illness, bad prognosis, pre-terminal) | Limited life expectancy conditions | Condition | Condition.category and Condition.code |
| Order for therapies indicating end of life care in past 90 days? | No | Look for patients with an existing order for therapies indicating end of life care written within past 90 days | Therapies indicating end of life care | ServiceRequest | ServiceRequest.status, ServiceRequest.intent, ServiceRequest.authoredOn, and ServiceRequest.code |
| Active cancer treatment? | No | See Active Cancer Treatment sub-routine | See Active Cancer Treatment sub-routine | ||
| Conditions Likely Terminal for opioid prescribing present? | No | Look for patients with active conditions in the value set end-of-life-conditions | Conditions likely terminal for opioid prescribing | Condition | Condition.category, Condition.code, and Condition.clinicalStatus |
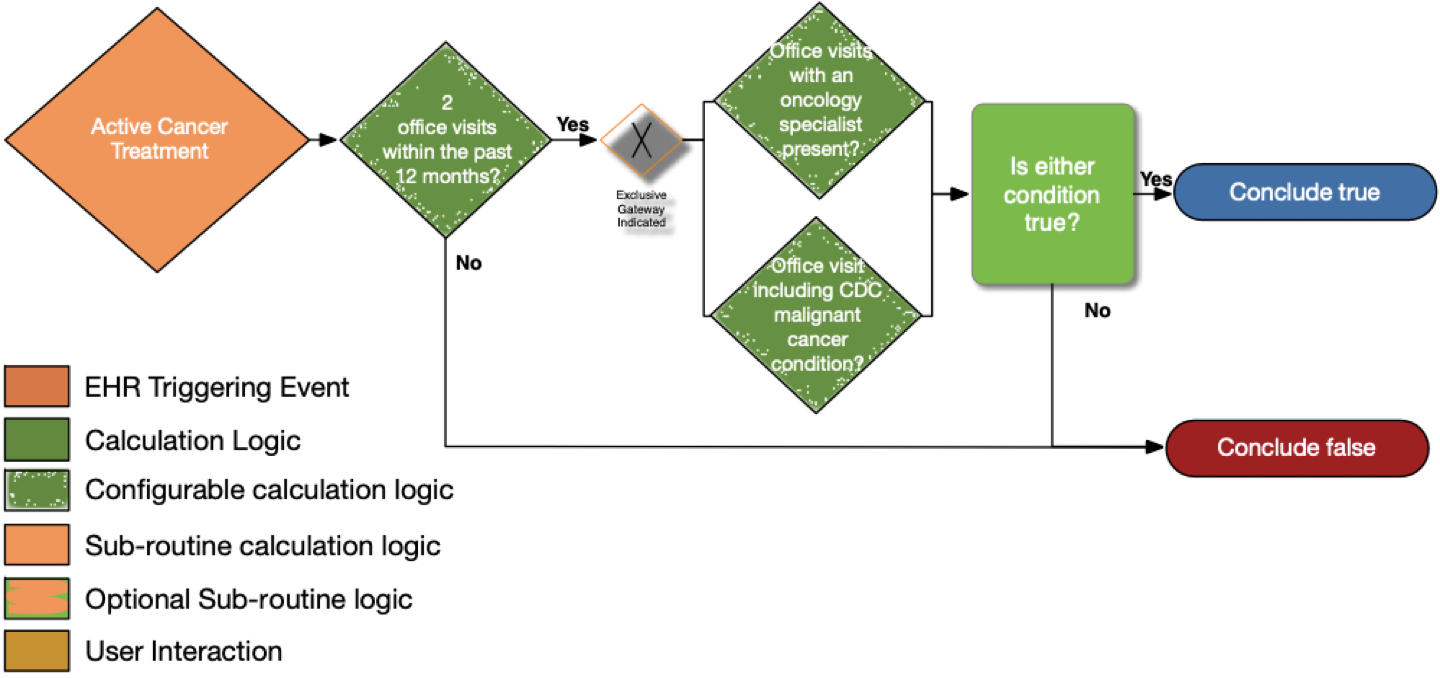
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Two office visits within the past 12 months? | No | Look for a minimum of two distinct encounters within 12 months of the date of the current visit for which each of the following is true: • the encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set |
Office Visit | Encounter | Encounter.class and Encounter.period.start |
| Office visits with an oncology specialist present? | No | • The encounter is performed by an oncologist as defined in the oncology specialty designations using the National Uniform Claim Committee (NUCC) classifications |
Oncology specialty designations (NUCC) | PractitionerRole and Encounter | PractitionerRole.specialty, Encounter.participant.type, and Encounter.participant.individual |
| Office visits including CDC malignant cancer condition? | No | • The encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set | CDC malignant cancer conditions | Condition and Encounter | Condition.category, Condition.code, and Encounter.diagnosis |
The following artifacts formalize the description of the logic and behavior defined by this recommendation.
| Resource | Type | Description |
|---|---|---|
| 2022 CDC Clinical Practice Guideline Recommendation #10 Patient View | PlanDefinition | Event-Condition-Action rule that implements behavior for 2022 CDC Clinical Practice Guideline Recommendation #10 Patient View |
| Recommendation #10 Patient View - urine drug testing when prescribing opioids for subacute or chronic pain | Library | Defines the data requirements to support evaluation of recommendation #10 Patient View |
| Opioid Terminology Management Knowledge-base Data (OMTK) Library | Library | CQL Library that provides logic for implementation of opioid management functionality including Milligram Morphine Equivalents (MME). |
| Opioid Terminology Management Knowledge-base (OMTK) Library | Library | CQL Library that provides logic for implementation of opioid management functionality including Milligram Morphine Equivalents (MME). |
| Common Opioid Decision Support Logic | Library | CQL Library that provides common logic for the recommendations |
| Common OpioidCDS Configuration Logic | Library | CQL Library that provides common configuration logic for the recommendations |
| Common OpioidCDS Routines Logic | Library | CQL Library that provides common routines logic for the recommendations |
| FHIRHelpers Conversion Logic | Library | CQL Library that defines functions to convert between FHIR data types and CQL system-defined types, as well as functions to support FHIRPath implementation |
| Description | CDS Hooks Request | Expected Response |
|---|---|---|
| Patient 18 or older. Patient is being prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RxNorm code 197696), once every 12 days for 30 days.
This triggers the message that urine drug screening is recommended with the following three feedback options: i. Perform the screening ii. Indicate that the prescription is not for subacute or chronic pain management and snooze for 3 months iii. Indicate that it is not applicable, log a comment and snooze for 3 months. |
Request JSON | Response JSON |
| Patient 18 or older. Patient is being prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RxNorm code 197696), once every 12 days for 30 days. Patient has condition indicating end of life (Carcinoma of endocrine pancreas). The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
IG © 2019+ Centers for Disease Control and Prevention (CDC). Package fhir.cdc.opioid-cds-r4#2022.1.0 based on FHIR 4.0.1. Generated 2024-04-22
Links: Table of
Contents |
License |
QA Report
| New Issue | Issues
| Version History |