2022 CDC Clinical Practice Guideline for Prescribing Opioids Implementation Guide
2022.1.0 - CI Build
2022 CDC Clinical Practice Guideline for Prescribing Opioids Implementation Guide, published by Centers for Disease Control and Prevention (CDC). This guide is not an authorized publication; it is the continuous build for version 2022.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/opioid-cds-r4/ and changes regularly. See the Directory of published versions
Recommendations #8 (2022 CDC Clinical Practice Guideline for Prescribing Opioids for Pain):
Before starting and periodically during continuation of opioid therapy, clinicians should evaluate risk for opioid-related harms and discuss risk with patients. Clinicians should work with patients to incorporate into the management plan strategies to mitigate risk, including offering naloxone (recommendation category: A; evidence type: 4).
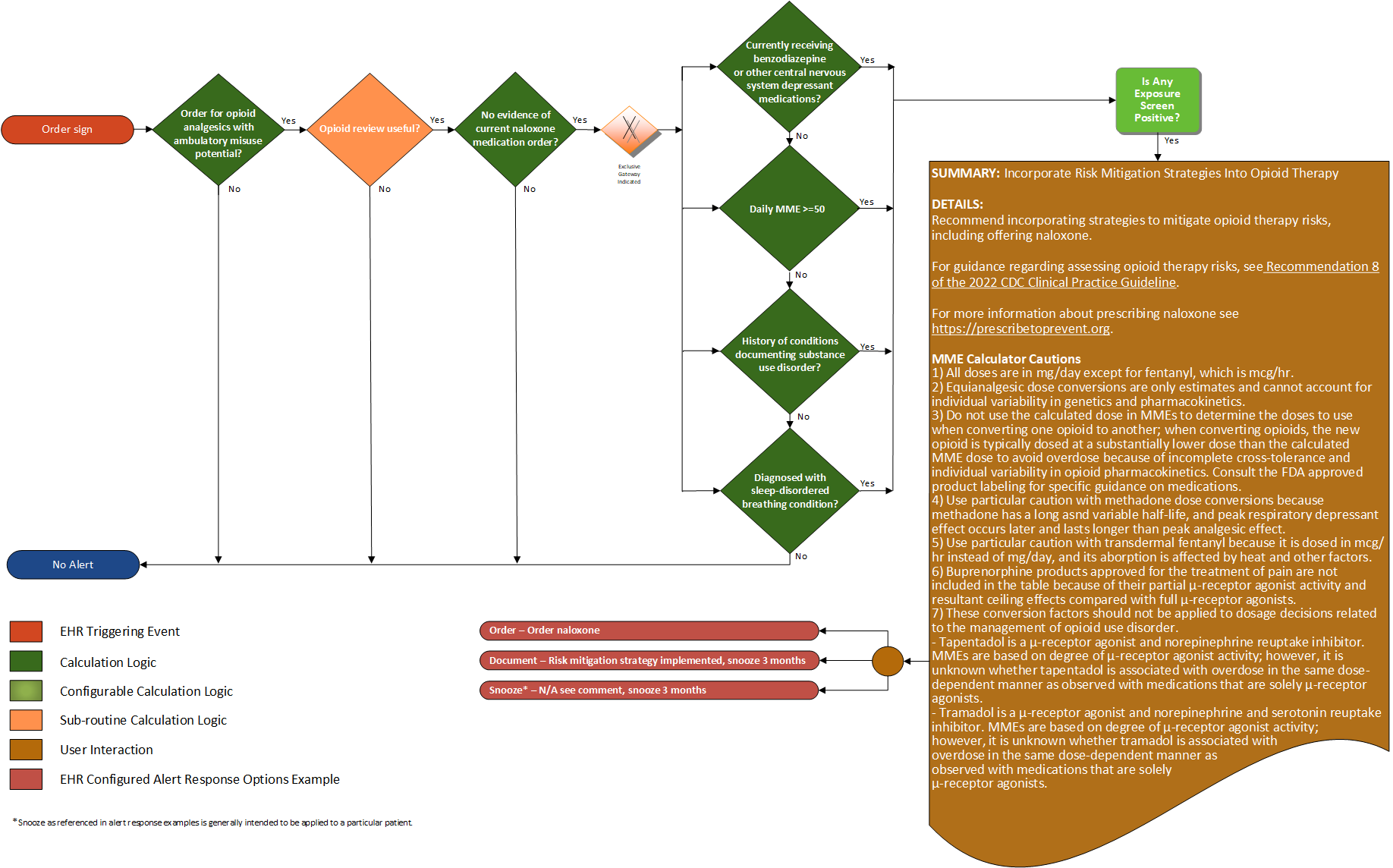
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Order for opioid analgesic with ambulatory misuse potential | Yes | Order for opioid analgesics with ambulatory misuse potential | Opioid analgesics with ambulatory misuse potential | MedicationRequest | MedicationRequest.medication, and MedicationRequest.category |
| Opioid review useful? | Yes | See Opioid Review Useful sub-routine | |||
| No evidence of current naloxone medications? | Yes | Find evidence of naloxone medication prescription (an active medication order for naloxone) | Naloxone medications | MedicationRequest | MedicationRequest.medication, and MedicationRequest.authoredOn |
| Currently receiving benzodiazepine or other central nervous system depressant medications? | Yes | Find evidence of benzodiazepine prescription (an active medication order for benzodiazepine or other central nervous system depressant medications) | Benzodiazepine or other central nervous system depressant medications | MedicationRequest | MedicationRequest.medication, MedicationRequest.category, and MedicationRequest.authoredOn |
| MME (morphine milligram equivalents > 50) | Yes | Determine MME from existing active prescriptions (recommendation 4 & 5), or future scope; dispensed medications, or patient-reported medications | MedicationRequest | MedicationRequest.medication, MedicationRequest.category, MedicationRequest.authoredOn, and MedicationRequest.dosageInstruction | |
| History of conditions documenting substance use disorder? | Yes | Find evidence of conditions documenting substance use in the problem list or past medical history, including history of overdose | Conditions documenting substance misuse disorder | Condition Procedure | Condition.code, Condition.category, and Procedure.code |
| Diagnosis with a sleep-disordered breathing condition? | Yes | Find documentation of an active sleep-disordered breathing condition | Conditions documenting sleep-disordered breathing | TBD | TBD |
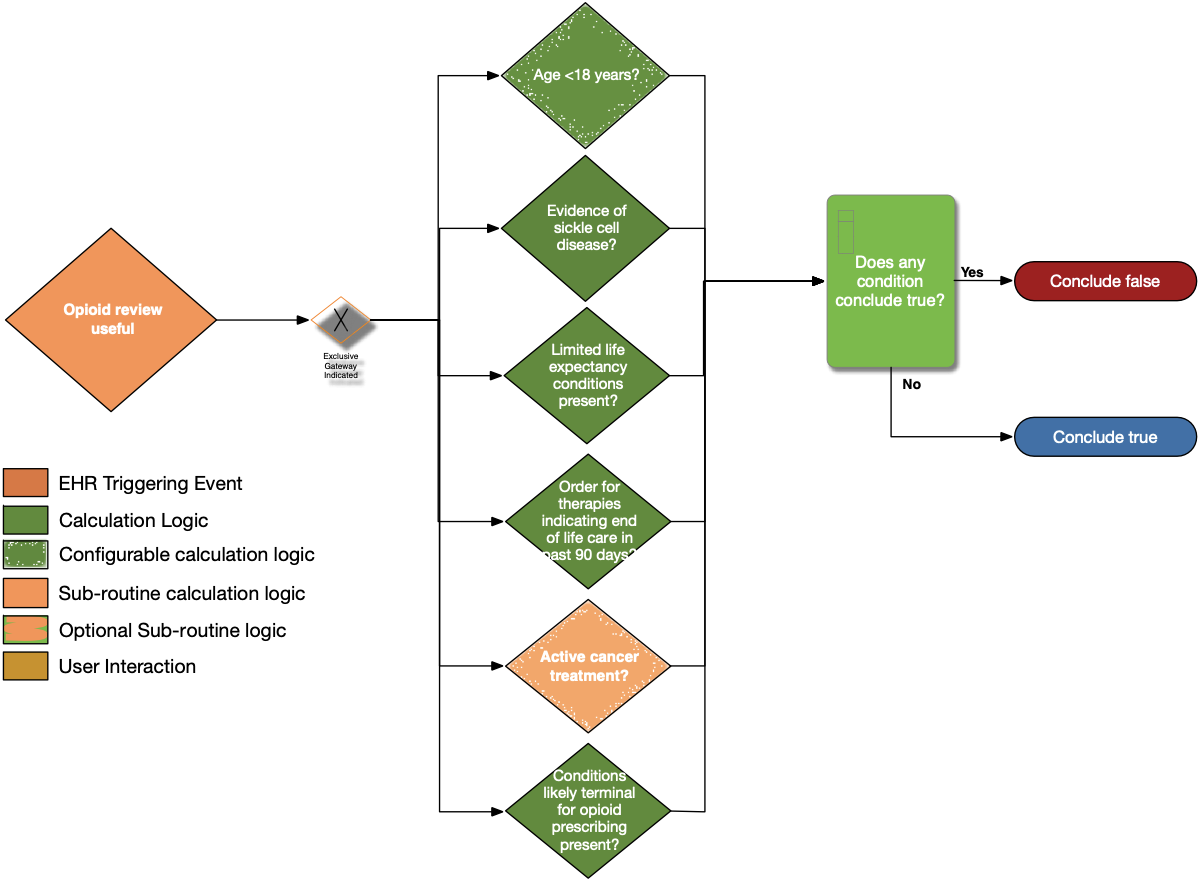
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Age < 18 years? | No | Calculate age from date of birth; exclude patients with age less than 18 years at the time of the prescription | Date of birth | Patient | Patient.birthDate |
| Evidence of sickle cell disease? | No | Look for patients with a diagnosis or problem list entry indicating sickle cell disease | Sickle cell disease condition | Condition | Condition.category, Condition.code, and Condition.clinicalStatus |
| Limited life expectancy conditions present? | No | Look for documented findings consistent with those listed in the limited life expectancy value set (terminal illness, bad prognosis, pre-terminal) | Limited life expectancy conditions | Condition | Condition.category and Condition.code |
| Order for therapies indicating end of life care in past 90 days? | No | Look for patients with an existing order for therapies indicating end of life care written within past 90 days | Therapies indicating end of life care | ServiceRequest | ServiceRequest.status, ServiceRequest.intent, ServiceRequest.authoredOn, and ServiceRequest.code |
| Active cancer treatment? | No | See Active Cancer Treatment sub-routine | See Active Cancer Treatment sub-routine | ||
| Conditions Likely Terminal for opioid prescribing present? | No | Look for patients with active conditions in the value set end-of-life-conditions | Conditions likely terminal for opioid prescribing | Condition | Condition.category, Condition.code, and Condition.clinicalStatus |
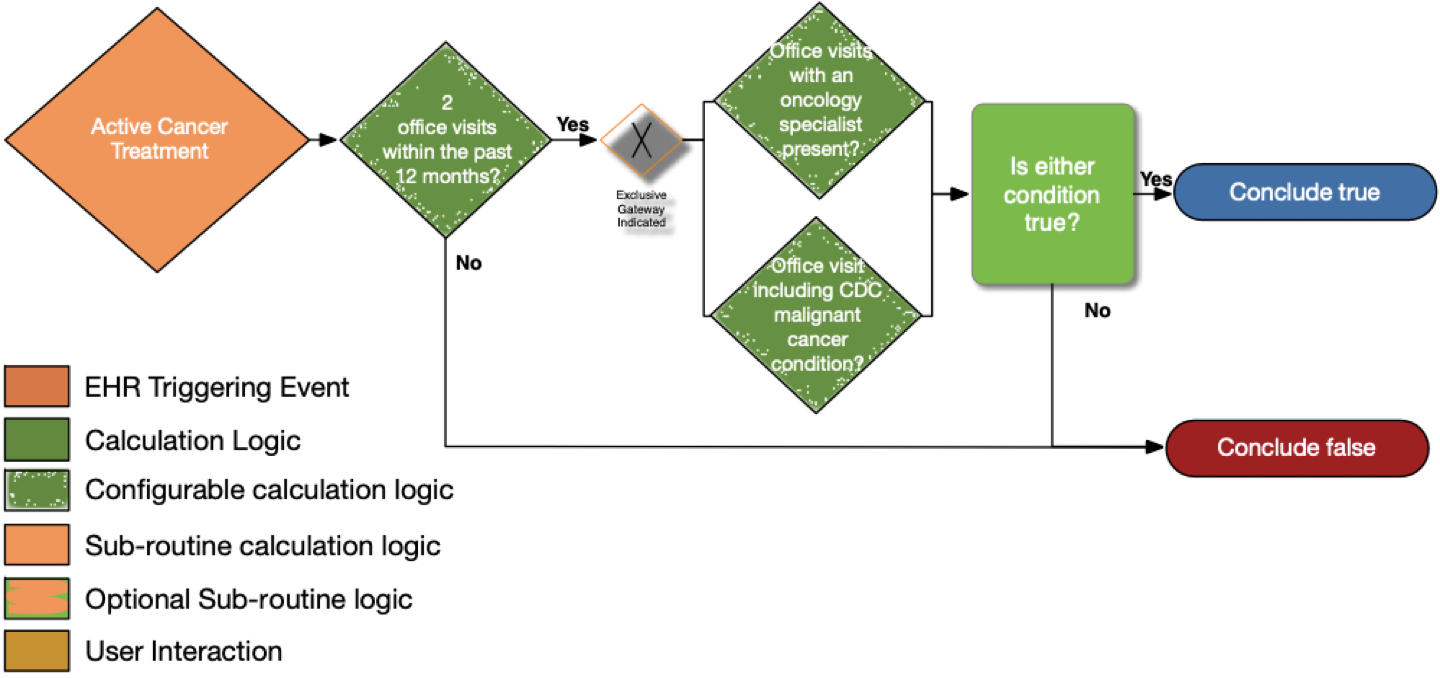
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Two office visits within the past 12 months? | No | Look for a minimum of two distinct encounters within 12 months of the date of the current visit for which each of the following is true: • the encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set |
Office Visit | Encounter | Encounter.class and Encounter.period.start |
| Office visits with an oncology specialist present? | No | • The encounter is performed by an oncologist as defined in the oncology specialty designations using the National Uniform Claim Committee (NUCC) classifications |
Oncology specialty designations (NUCC) | PractitionerRole and Encounter | PractitionerRole.specialty, Encounter.participant.type, and Encounter.participant.individual |
| Office visits including CDC malignant cancer condition? | No | • The encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set | CDC malignant cancer conditions | Condition and Encounter | Condition.category, Condition.code, and Encounter.diagnosis |
The following artifacts formalize the description of the logic and behavior defined by this recommendation.
| Resource | Type | Description |
|---|---|---|
| 2022 CDC Clinical Practice Guideline Recommendation #8 | PlanDefinition | Event-Condition-Action rule that implements behavior for 2022 CDC Clinical Practice Guideline Recommendation #8 |
| Recommendation #8 - risk factors for opioid-related harms before and during opioid therapy | Library | Defines the data requirements to support evaluation of recommendation #8 |
| Opioid Terminology Management Knowledge-base Data (OMTK) Library | Library | CQL Library that provides logic for implementation of opioid management functionality including Milligram Morphine Equivalents (MME). |
| Opioid Terminology Management Knowledge-base (OMTK) Library | Library | CQL Library that provides logic for implementation of opioid management functionality including Milligram Morphine Equivalents (MME). |
| Common Opioid Decision Support Logic | Library | CQL Library that provides common logic for the recommendations |
| Common OpioidCDS Configuration Logic | Library | CQL Library that provides common configuration logic for the recommendations |
| Common OpioidCDS Routines Logic | Library | CQL Library that provides common routines logic for the recommendations |
| FHIRHelpers Conversion Logic | Library | CQL Library that defines functions to convert between FHIR data types and CQL system-defined types, as well as functions to support FHIRPath implementation |
| Description | CDS Hooks Request | Expected Response |
|---|---|---|
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 10 days for 30 days. This will trigger the message "Incorporate into the management plan strategies to mitigate risk; including considering offering naloxone when factors that increase risk for opioid overdose are present." Consider offering naloxone given following risk factor(s) for opioid overdose: Average MME (54.000000 'mg/d') >= 50 mg/day. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. Patient has also been prescribed Temazepam 20 MG Oral Tablet (RXNorm 104693) 1 tablet per 1 day for 30 days. This will trigger the message “Incorporate into the management plan strategies to mitigate risk; including considering offering naloxone when factors that increase risk for opioid overdose are present. Consider offering naloxone given following risk factor(s) for opioid overdose: concurrent use of benzodiazepine. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. Patient has also been prescribed Naloxone Hydrochloride 0.02 MG/ML Injectable Solution (RXNorm 1191212) 1ml per 1 day for 30 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 72 HR Fentanyl 0.075 MG/HR Transdermal System (RXNorm 197696) 1 patch per 12 days for 30 days. This will trigger the message "Incorporate into the management plan strategies to mitigate risk; including considering offering naloxone when factors that increase risk for opioid overdose are present" Consider offering naloxone given following risk factor(s) for opioid overdose: history of alcohol or drug abuse. | Request JSON | Response JSON |
IG © 2019+ Centers for Disease Control and Prevention (CDC). Package fhir.cdc.opioid-cds-r4#2022.1.0 based on FHIR 4.0.1. Generated 2024-04-22
Links: Table of
Contents |
License |
QA Report
| New Issue | Issues
| Version History |