Pharmaceutical Quality (Industry), published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 1.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/uv-dx-pq/ and changes regularly. See the Directory of published versions
Details about compatibility studies and results.
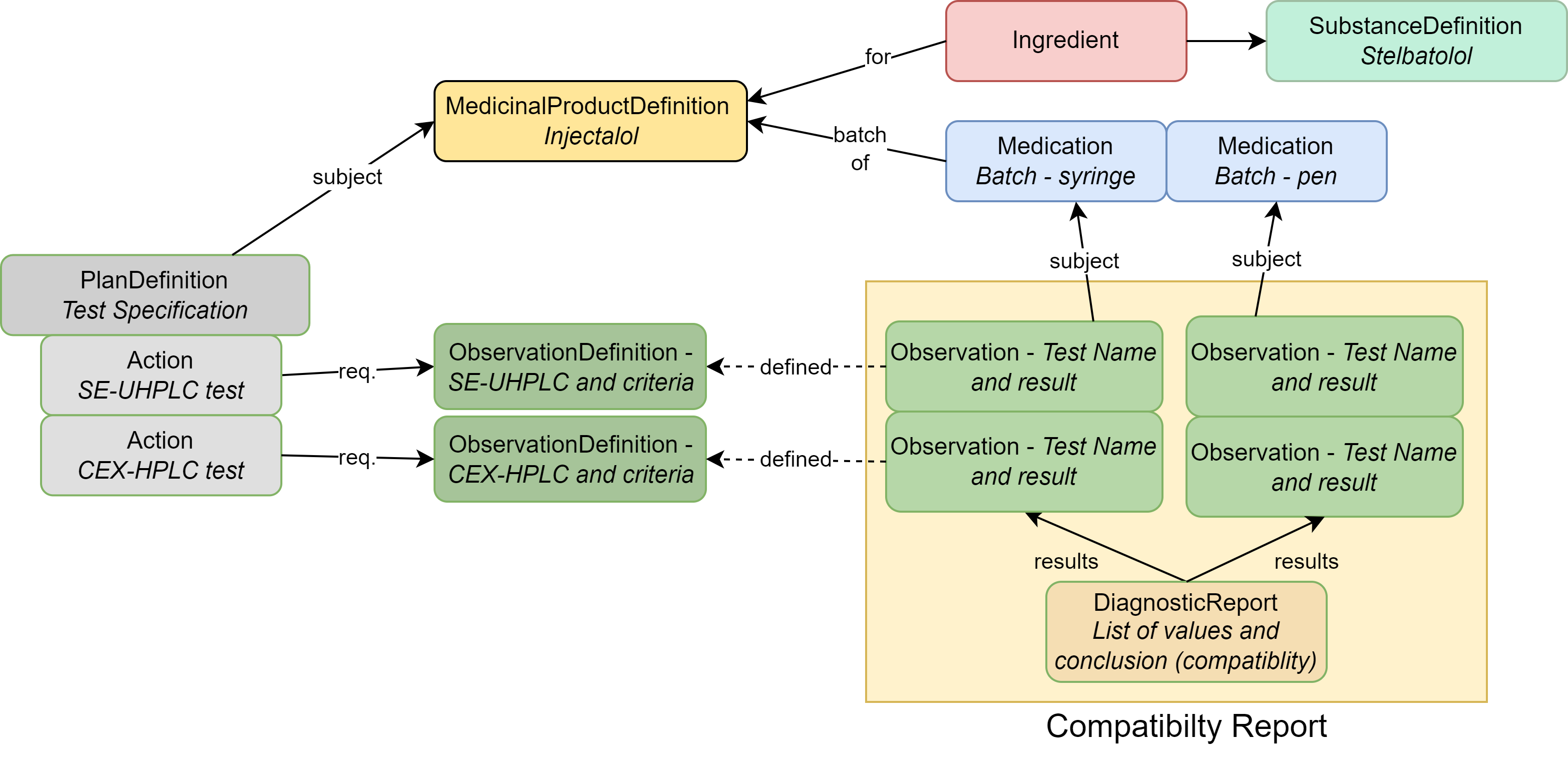 |
| MedicinalProductDefinition | The drug product (Stelbat tablets, 20mg) |
| Medication | Describes the batches that underwent testing |
| Ingredient | The active ingredient (stelbatalol) or the ingredients that make up the drug product |
| SubstanceDefinition | Chemical or biological details about substance(s) associated with the ingredient |
| PlanDefinition | Describes the compatibility analysis protocol |
| ObservationDefinition | Each individual test and acceptance criteria; also used to group tests |
| Observation | The results of a specific test mentioned in the ObservationDefinition |
| DiagnosticReport | Contains all results as a group and captures conclusions |
CTD section samples (PDF):
XML and JSON examples of synthetic quality data:
HTML rendering of synthetic quality data: