SPL Mapping FHIR R5 Implementation Guide, published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 0.2.8 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/fhir-spl/ and changes regularly. See the Directory of published versions
| Page standards status: Informative |
This page details the following use cases that all use profiles based on the Medicinal Product Definition resources:
Here is a high level diagram that shows the generic use of the profiles for these use cases:
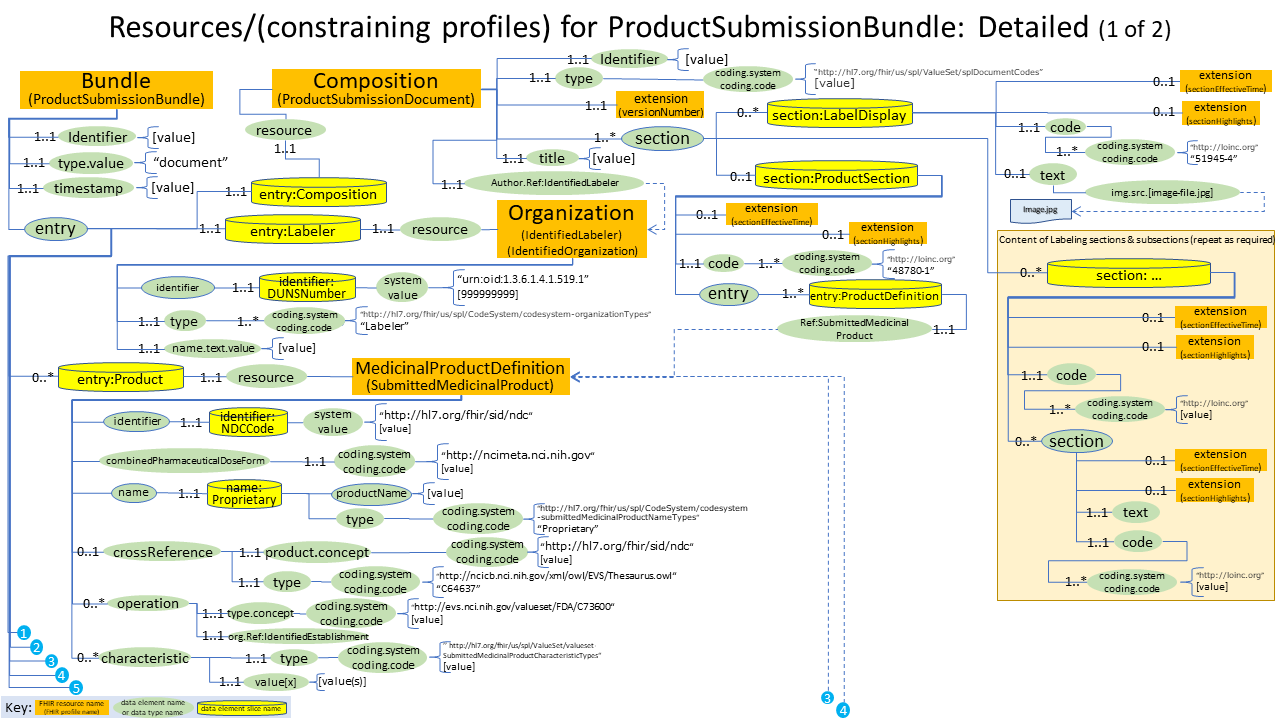
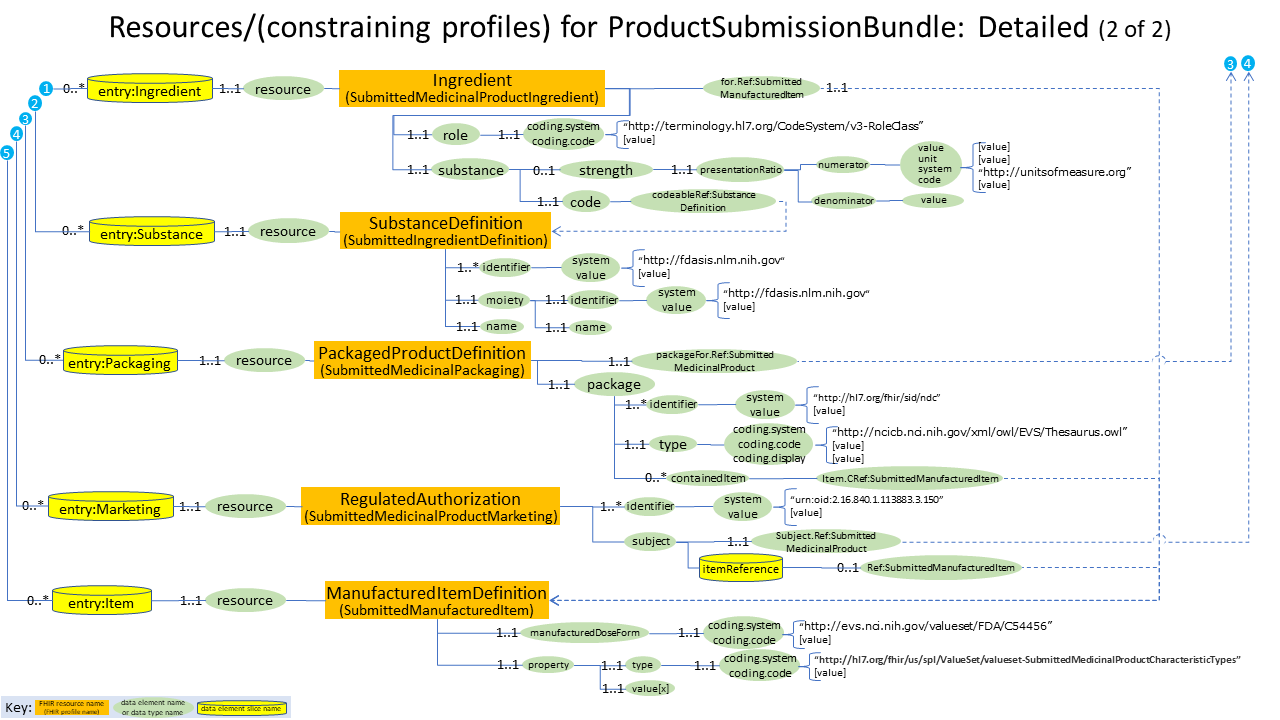
Here is a detailed diagram that shows the profiles used for drug label submission: