SPL Mapping FHIR R5 Implementation Guide, published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 0.2.8 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/fhir-spl/ and changes regularly. See the Directory of published versions
Document Details
Profile: ProductSubmissionBundle
Final Document at 2021-12-13 by Organization Immunex Corporation for
Document Content
WARNING: SERIOUS INFECTIONS and MALIGNANCIES
See full prescribing information for complete boxed warning.
SERIOUS INFECTIONS
MALIGNANCIES
SERIOUS INFECTIONS
Patients treated with Enbrel are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1) and Adverse Reactions (6)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Enbrel should be discontinued if a patient develops a serious infection or sepsis.
Reported infections include:
The risks and benefits of treatment with Enbrel should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with Enbrel, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCIES
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF-blockers, including Enbrel.
Enbrel is a tumor necrosis factor (TNF) blocker indicated for the treatment of:
Enbrel is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis (RA). Enbrel can be initiated in combination with methotrexate (MTX) or used alone.
Enbrel is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis (JIA) in patients ages 2 and older.
Enbrel is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in patients with psoriatic arthritis (PsA). Enbrel can be used with or without methotrexate.
Enbrel is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis (AS).
Enbrel is indicated for the treatment of patients 4 years or older with chronic moderate to severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
Enbrel is administered by subcutaneous injection.
| Patient Population | Recommended Dose and Frequency |
|---|---|
| Adult RA and PsA (2.1) | 50 mg once weekly with or without methotrexate (MTX) |
| AS (2.1) | 50 mg once weekly |
| Adult PsO (2.1) | 50 mg twice weekly for 3 months, followed by 50 mg once weekly |
| Pediatric PsO or JIA (2.2) | 0.8 mg/kg weekly, with a maximum of 50 mg per week |
Administration of one 50 mg Enbrel single-dose prefilled syringe, one single-dose prefilled Enbrel SureClick autoinjector, or one Enbrel Mini single-dose prefilled cartridge (for use with the AutoTouch reusable autoinjector only), provides a dose equivalent to two 25 mg Enbrel single-dose prefilled syringes, two 25 mg single-dose vials, or two multiple-dose vials of lyophilized Enbrel, when multiple-dose vials are reconstituted and administered as recommended.
Enbrel is administered by subcutaneous injection.
| Patient Population | Recommended Dosage Strength and Frequency |
|---|---|
| Adult RA, AS, and PsA | 50 mg weekly |
| Adult PsO | Starting Dose: 50 mg twice weekly for 3 months Maintenance Dose: 50 mg once weekly |
See the Enbrel (etanercept) "Instructions for Use" insert for detailed information on injection site selection and dose administration [see Dosage and Administration (2.3) and Patient Counseling Information (17)].
Adult Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients
Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Enbrel.
Based on a study of 50 mg Enbrel twice weekly in patients with RA that suggested higher incidence of adverse reactions but similar American College of Rheumatology (ACR) response rates, doses higher than 50 mg per week are not recommended.
Adult Plaque Psoriasis Patients
In addition to the 50 mg twice weekly recommended starting dose, starting doses of 25 mg or 50 mg per week were shown to be efficacious. The proportion of responders was related to Enbrel dosage [see Clinical Studies (14.5)].
Enbrel is administered by subcutaneous injection.
| Pediatric Patients Weight | Recommended Dose |
|---|---|
| 63 kg (138 pounds) or more | 50 mg weekly |
| Less than 63 kg (138 pounds) | 0.8 mg/kg weekly |
To achieve pediatric doses other than 25 mg or 50 mg, use Enbrel solution in a single-dose vial or reconstituted lyophilized powder in a multiple-dose vial.
Doses of Enbrel higher than those described in Table 2 have not been studied in pediatric patients.
In JIA patients, glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Enbrel.
Enbrel is intended for use under the guidance and supervision of a physician. Patients may self-inject when deemed appropriate and if they receive medical follow-up, as necessary. Patients should not self-administer until they receive proper training in how to prepare and administer the correct dose. Administer injections subcutaneously in the thigh, abdomen or outer area of the upper arm.
The following components contain dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex: the needle cover of the prefilled syringe, the needle cover within the white cap of the SureClick autoinjector, and the needle cover within the purple cap of the Enbrel Mini cartridge [see Warnings and Precautions (5.7)].
The Enbrel (etanercept) "Instructions for Use" insert for each presentation contains more detailed instructions on injection site selection and the preparation of Enbrel.
Preparation of Enbrel Single-dose Prefilled Syringe
For a more comfortable injection, leave Enbrel prefilled syringes at room temperature for about 15 to 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose prefilled syringe, check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of Enbrel Single-dose Prefilled SureClick Autoinjector
Leave the autoinjector at room temperature for at least 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of Enbrel Single-dose Vial
For a more comfortable injection, leave Enbrel vial(s) at room temperature for at least 30 minutes before injecting. DO NOT remove the vial cap while allowing the vial to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose vial, administer the correct dose of solution using the following recommended materials:
Two vials may be required to administer the total prescribed dose. Use the same syringe for each vial. The vial does not contain preservatives; therefore discard unused portions.
Preparation of Enbrel Lyophilized Powder in a Multiple-dose Vial
Enbrel lyophilized powder should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), giving a solution of 1 mL containing 25 mg of Enbrel.
A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25-gauge needle should be used for reconstituting and withdrawing Enbrel, and the supplied "Mixing Date:" sticker should be attached to the vial and the date of reconstitution entered. Reconstituted solution must be refrigerated at 36°F to 46°F (2°C to 8°C) and used within 14 days. Discard reconstituted solution after 14 days because product stability and sterility cannot be assured after 14 days. DO NOT store reconstituted Enbrel solution at room temperature.
For a more comfortable injection, leave the Enbrel dose tray at room temperature for about 15 to 30 minutes before injecting.
If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the Enbrel vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the Enbrel vial. If using a 25-gauge needle to reconstitute and withdraw Enbrel, the diluent should be injected very slowly into the Enbrel vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the Enbrel vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
Generally, dissolution of Enbrel takes less than 10 minutes. Do not use the solution if discolored or cloudy, or if particulate matter remains.
Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25-gauge needle from the syringe. Attach a 27-gauge needle to inject Enbrel.
The contents of one vial of Enbrel solution should not be mixed with, or transferred into, the contents of another vial of Enbrel. No other medications should be added to solutions containing Enbrel, and do not reconstitute Enbrel with other diluents. Do not filter reconstituted solution during preparation or administration.
Preparation of Enbrel Mini® single-dose prefilled cartridge using the AutoTouch® reusable autoinjector
Leave Enbrel Mini single-dose prefilled cartridge at room temperature for at least 30 minutes before injecting. DO NOT remove the purple cap while allowing the cartridge to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
To use AutoTouch reusable autoinjector, open the door by pushing the door button and inserting Enbrel Mini single-dose prefilled cartridge into AutoTouch. When inserted correctly, Enbrel Mini single-dose prefilled cartridge will slide freely and completely into the door. Close the door and AutoTouch reusable autoinjector is ready for injection.
Prior to initiating Enbrel and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection [see Warnings and Precautions (5.1)].
PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-455-04
Enbrel®
etanercept
25 mg/0.5 mL
Single-Dose Prefilled Syringe
25 mg/0.5 mL
Attention: Not for use in pediatric patients under 31 kg (68 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 single-dose prefilled syringes, 1 package
insert with attached Medication Guide) are intended to be
dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required
for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320

PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-435-04
Enbrel®
etanercept
50 mg/mL
Single-Dose Prefilled Syringe
50 mg/mL
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 single-dose prefilled syringes, 1 package
insert with attached Medication Guide) are intended to be
dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required
for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320

PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-010-04
Enbrel®
etanercept
25 mg/0.5 mL
Single-Dose Prefilled Syringe
25 mg/0.5 mL
Attention: Not for use in pediatric patients under 31 kg (68 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 single-dose prefilled syringes, 1 package
insert with attached Medication Guide) are intended to be
dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required
for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320

PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-021-04
Enbrel®
etanercept
50 mg/mL
Single-Dose Prefilled Syringe
50 mg/mL
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 single-dose prefilled syringes, 1 package
insert with attached Medication Guide) are intended to be
dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required
for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320

PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Autoinjectors
NDC 58406-445-04
Enbrel®
etanercept
SureClick® Autoinjector
50 mg/mL
Single-Dose Prefilled Autoinjector
50 mg/mL
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton Contents (4 prefilled SureClick® Autoinjectors, 1 package insert with
attached Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320-1799

PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Autoinjectors
NDC 58406-032-04
Enbrel®
etanercept
SureClick® Autoinjector
50 mg/mL
Single-Dose Prefilled Autoinjector
50 mg/mL
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton Contents (4 prefilled SureClick® Autoinjectors, 1 package insert with
attached Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320-1799

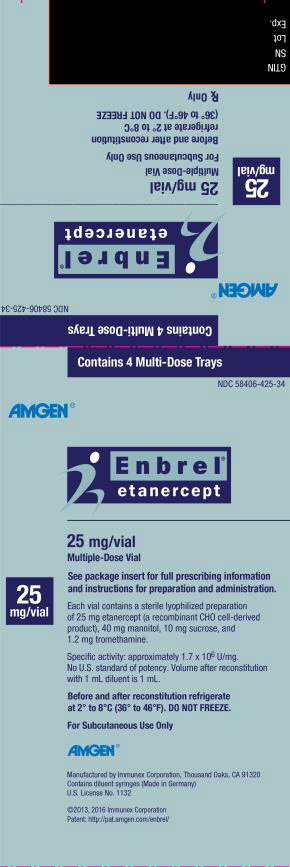
PRINCIPAL DISPLAY PANEL
Contains 4 Multi-Dose Trays
NDC 58406-425-34
AMGEN®
Enbrel®
etanercept
25 mg/vial
Multiple-Dose Vial
See package insert for full prescribing information
and instructions for preparation and administration.
25 mg/vial
Each vial contains a sterile lyophilized preparation
of 25 mg etanercept (a recombinant CHO cell-derived
product), 40 mg mannitol, 10 mg sucrose, and
1.2 mg tromethamine.
Specific activitiy: approximately 1.7 x 106 U/mg.
No U.S. standard of potency. Volume after reconstitution
with 1 mL diluent is 1 mL.
Before and after reconstitution refrigerate
at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
For Subcutaneous Use Only
AMGEN®
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
Contains diluent syringes (Made in Germany)
U.S. License No. 1132
©2013, 2016 Immunex Corporation
Patent: http://pat.amgen.com/enbrel/
PRINCIPAL DISPLAY PANEL
Contains 4 Single-dose prefilled cartridges
NDC 58406-456-04
Enbrel®
etanercept
50 mg/mL
Enbrel Mini™ prefilled cartridge
50 mg/mL
Single-dose prefilled cartridge
For use with AutoTouch™ reusuable autoinjector only
Attention: Not for use in pediatric patient under 138 lbs.
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2°C to 8°C (36° to 46°F). DO NOT FREEZE. DO NOT SHAKE.
Carton contents (4 prefilled cartridges, 1 package insert with attached
Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains
Dry Natural Rubber.
Do not Reuse
CAUTION, See package insert
for full prescribing information
and Instructions for Use
Rx Only
AMGEN®

Contains 4 Single-dose prefilled cartridges
NDC 58406-044-04
Enbrel®
etanercept
50
mg/mL
Enbrel Mini® prefilled cartridge
50 mg/mL
Single-dose prefilled cartridge
For use with all AutoTouch® reusable autoinjectors only
Attention: Not for use in pediatric patient under 138 lbs.
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE. DO NOT SHAKE.
Carton contents (4 prefilled cartridges, 1 package insert with attached
Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains
Dry Natural Rubber.
Do not Reuse
CAUTION, See package insert
for full prescribing information
and Instructions for Use
Rx Only
AMGEN®

PRINCIPAL DISPLAY PANEL
Contains 4 Single-dose prefilled Autoinjectors
NDC 58406-446-04
Enbrel®
etanercept
50 mg/mL
SureClick® 2.0 Autoinjector
Enhanced Features
see instructions for use
50 mg/mL
Single-dose Prefilled Autoinjector
Attention: Not for use in pediatric patients under 63 kg (138 pounds)
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 prefilled SureClick® 2.0 Autoinjectors, 1 package insert with
attached Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320-1799
Marketed by Amgen Inc.

PRINCIPAL DISPLAY PANEL
NDC 58406-470-01
AMGEN®
AutoTouch® reusable autoinjector
For use with Enbrel Mini® (etanercept)
For use with Enbrel Mini® (etanercept) single-dose prefilled cartridge
Contains 1 AutoTouch® reusable autoinjector
For Subcutaneous Use Only
Store at room temperature.
IP52 – This package will resisit drops of water and dust.
Do Not Use if Package is Damaged
Type BF Applied Part
Exp: Expiry Date
Follow instructions for use
Rx Only

4 Single-Dose Vials, each vial is 25 mg/0.5mL
NDC 58406-055-04
AMGEN®
Enbrel®
etanercept
Injection
25 mg/0.5 mL
Single-Dose Vial – Discard unused portion
For Subcutaneous Use Only.
Sterile Solution - No Preservative.
Refrigerate at 2° to 8°C (36° to 46°F). Do Not Freeze or Shake.
Protect from Light.
25
mg
U.S. License No. 1132
Manufactured by Immunex Corporation
Thousand Oaks, CA 91320-1799 U.S.A.
© 2019 Immunex Corporation
Rx Only

Entry 2 - fullUrl = http://example.org/Organization/ImmunexCorporation
Resource Organization:
Profile: IdentifiedLabeler
identifier: Data Universal Numbering System (DUNS) Number/028134799
type: An organization that submits product labels.
name: Immunex Corporation
Entry 3 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel435Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-435
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2005-10-06 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 4 - fullUrl = http://example.org/ManufacturedItemDefinition/Enbrel50mgSolution
Resource ManufacturedItemDefinition:
Profile: SubmittedManufacturedItem
status: Active
manufacturedDoseForm: SOLUTION
Entry 5 - fullUrl = http://example.org/Ingredient/Enbrel50mgSolutionActiveIngredient
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: active ingredient - basis of strength
substance
Codes
Reference SubstanceDefinition: identifier = http://fdasis.nlm.nih.gov#Unique Ingredient Identifier (UNII)#OP401G7OJC Strengths
Presentation[x] 50 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 6 - fullUrl = http://example.org/SubstanceDefinition/EnbrelIngredientDefinition
Resource SubstanceDefinition:
Profile: SubmittedIngredientDefinition
identifier: Unique Ingredient Identifier (UNII)/OP401G7OJC
Moieties
Identifier Name Unique Ingredient Identifier (UNII)/OP401G7OJC ETANERCEPT Names
Name ETANERCEPT
Entry 7 - fullUrl = http://example.org/Ingredient/Enbrel50mgSolutionIngredient1
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept ARGININE HYDROCHLORIDE Strengths
Presentation[x] 0.025 mmol (Details: UCUM codemmol = 'mmol')/1 mL (Details: UCUM codemL = 'mL')
Entry 8 - fullUrl = http://example.org/Ingredient/Enbrel50mgSolutionIngredient2
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept SODIUM CHLORIDE Strengths
Presentation[x] 0.12 mmol (Details: UCUM codemmol = 'mmol')/1 mL (Details: UCUM codemL = 'mL')
Entry 9 - fullUrl = http://example.org/Ingredient/Enbrel50mgSolutionIngredient3
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept SUCROSE Strengths
Presentation[x] 10 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 10 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel435Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2005-10-05 --> (ongoing) packaging
identifier: National drug codes/58406-435-04
type: CARTON
packaging
identifier: National drug codes/58406-435-01
type: SYRINGE
quantity: 4
Properties
Type Value[x] Combination Product Type Type 0: Not a Combination Product containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 11 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel435Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 12 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel445Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-445
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2005-11-10 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 13 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel445Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2005-11-10 --> (ongoing) packaging
identifier: National drug codes/58406-445-04
type: CARTON
packaging
identifier: National drug codes/58406-445-01
type: SYRINGE
quantity: 4
Properties
Type Value[x] Combination Product Type Type 0: Not a Combination Product containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 14 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel445Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 15 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel425Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-425
MarketingStatuses
Status DateRange active 2003-01-02 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 16 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel425Part1Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-424
MarketingStatuses
Status DateRange active 2003-01-02 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 17 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel425Part1Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
packageFor: MedicinalProductDefinition: identifier = http://hl7.org/fhir/sid/ndc#National drug codes#58406-424
packaging
identifier: National drug codes/58406-424-01
type: VIAL
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 18 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel425Part1Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795subject: MedicinalProductDefinition: identifier = http://hl7.org/fhir/sid/ndc#National drug codes#58406-424
type: NDA
region: United States of America
Entry 19 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel425Part2Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-910
MarketingStatuses
Status DateRange active 2003-01-02 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 20 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel425Part2Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
packageFor: MedicinalProductDefinition: identifier = http://hl7.org/fhir/sid/ndc#National drug codes#58406-910
packaging
identifier: National drug codes/58406-910-01
type: SYRINGE
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 21 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel425Part2Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795subject: MedicinalProductDefinition: identifier = http://hl7.org/fhir/sid/ndc#National drug codes#58406-910
type: NDA
region: United States of America
Entry 22 - fullUrl = http://example.org/ManufacturedItemDefinition/EnbrelPowder
Resource ManufacturedItemDefinition:
Profile: SubmittedManufacturedItem
status: Active
manufacturedDoseForm: INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
Entry 23 - fullUrl = http://example.org/Ingredient/EnbrelPowderActiveIngredient
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
role: active ingredient - basis of strength
substance
Codes
Reference SubstanceDefinition: identifier = http://fdasis.nlm.nih.gov#Unique Ingredient Identifier (UNII)#OP401G7OJC Strengths
Presentation[x] 25 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 24 - fullUrl = http://example.org/Ingredient/EnbrelPowderIngredient1
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
role: inactive ingredient
substance
Codes
Concept MANNITOL Strengths
Presentation[x] 40 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 25 - fullUrl = http://example.org/Ingredient/EnbrelPowderIngredient2
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
role: inactive ingredient
substance
Codes
Concept SUCROSE Strengths
Presentation[x] 10 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 26 - fullUrl = http://example.org/Ingredient/EnbrelPowderIngredient3
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
role: inactive ingredient
substance
Codes
Concept TROMETHAMINE Strengths
Presentation[x] 1.2 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 27 - fullUrl = http://example.org/ManufacturedItemDefinition/EnbrelSterileSolution
Resource ManufacturedItemDefinition:
Profile: SubmittedManufacturedItem
status: Active
manufacturedDoseForm: SOLUTION
Entry 28 - fullUrl = http://example.org/Ingredient/EnbrelSterileSolutionIngredient1
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept BENZYL ALCOHOL Strengths
Presentation[x] 9.93 mg (Details: UCUM codemg = 'mg')/1 mL (Details: UCUM codemL = 'mL')
Entry 29 - fullUrl = http://example.org/Ingredient/EnbrelSterileSolutionIngredient2
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept WATER Strengths
Presentation[x] 1 mL (Details: UCUM codemL = 'mL')/1 mL (Details: UCUM codemL = 'mL')
Entry 30 - fullUrl = http://example.org/ManufacturedItemDefinition/EnbrelKit
Resource ManufacturedItemDefinition:
Profile: SubmittedManufacturedItem
status: Active
manufacturedDoseForm: KIT
Entry 31 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel425Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
packageFor: MedicinalProductDefinition: identifier = http://hl7.org/fhir/sid/ndc#National drug codes#58406-425
MarketingStatuses
Status DateRange active 2003-10-02 --> (ongoing) packaging
identifier: National drug codes/58406-425-34
type: CARTON
packaging
identifier: National drug codes/58406-425-41
type: KIT
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = KIT amount: 1 1 (Details: UCUM code1 = '1')
packaging
Packaged Product Reference: PackagedProductDefinition
identifier: National drug codes/58406-424-01
type: VIAL
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
packaging
Packaged Product Reference: PackagedProductDefinition
identifier: National drug codes/58406-910-01
type: SYRINGE
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 32 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel425Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795subject: MedicinalProductDefinition: identifier = http://hl7.org/fhir/sid/ndc#National drug codes#58406-425
type: NDA
region: United States of America
Entry 33 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel455Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-455
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2005-11-10 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 34 - fullUrl = http://example.org/ManufacturedItemDefinition/Enbrel25mgSolution
Resource ManufacturedItemDefinition:
Profile: SubmittedManufacturedItem
status: Active
manufacturedDoseForm: SOLUTION
Entry 35 - fullUrl = http://example.org/Ingredient/Enbrel25mgSolutionActiveIngredient
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: active ingredient - basis of strength
substance
Codes
Reference SubstanceDefinition: identifier = http://fdasis.nlm.nih.gov#Unique Ingredient Identifier (UNII)#OP401G7OJC Strengths
Presentation[x] 25 mg (Details: UCUM codemg = 'mg')/0.5 mL (Details: UCUM codemL = 'mL')
Entry 36 - fullUrl = http://example.org/Ingredient/Enbrel25mgSolutionIngredient1
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept ARGININE HYDROCHLORIDE Strengths
Presentation[x] 0.013 mmol (Details: UCUM codemmol = 'mmol')/0.5 mL (Details: UCUM codemL = 'mL')
Entry 37 - fullUrl = http://example.org/Ingredient/Enbrel25mgSolutionIngredient2
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept SODIUM CHLORIDE Strengths
Presentation[x] 0.06 mmol (Details: UCUM codemmol = 'mmol')/0.5 mL (Details: UCUM codemL = 'mL')
Entry 38 - fullUrl = http://example.org/Ingredient/Enbrel25mgSolutionIngredient3
Resource Ingredient:
Profile: SubmittedMedicinalProductIngredient
status: Active
for: ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION
role: inactive ingredient
substance
Codes
Concept SUCROSE Strengths
Presentation[x] 5 mg (Details: UCUM codemg = 'mg')/0.5 mL (Details: UCUM codemL = 'mL')
Entry 39 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel455Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2005-11-10 --> (ongoing) packaging
identifier: National drug codes/58406-455-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-455-01
type: SYRINGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 0.5 mL (Details: UCUM codemL = 'mL')
Entry 40 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel455Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 41 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel456Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-456
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2017-09-29 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 42 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel456Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2017-09-29 --> (ongoing) packaging
identifier: National drug codes/58406-456-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-456-01
type: CARTRIDGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 43 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel456Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 44 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel446Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-446
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2017-10-20 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 45 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel446Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2017-10-20 --> (ongoing) packaging
identifier: National drug codes/58406-446-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-446-01
type: SYRINGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 46 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel446Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 47 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel021Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-021
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 48 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel021Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) packaging
identifier: National drug codes/58406-021-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-021-01
type: SYRINGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 49 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel021Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 50 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel032Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-032
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 51 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel032Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) packaging
identifier: National drug codes/58406-032-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-032-01
type: SYRINGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 52 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel032Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 53 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel010Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-010
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 54 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel010Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) packaging
identifier: National drug codes/58406-010-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-010-01
type: SYRINGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 0.5 mL (Details: UCUM codemL = 'mL')
Entry 55 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel010Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 56 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel044Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-044
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 57 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel044Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2019-06-07 --> (ongoing) packaging
identifier: National drug codes/58406-044-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-044-01
type: CARTRIDGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 1 mL (Details: UCUM codemL = 'mL')
Entry 58 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel044Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America
Entry 59 - fullUrl = http://example.org/MedicinalProductDefinition/Enbrel055Definition
Resource MedicinalProductDefinition:
Profile: SubmittedMedicinalProduct
identifier: National drug codes/58406-055
route: SUBCUTANEOUS
MarketingStatuses
Status DateRange active 2020-03-05 --> (ongoing) name
productName: ENBREL
type: Proprietary Name
name
productName: etanercept
type: Generic Name (Non-Proprietary)
Entry 60 - fullUrl = http://example.org/PackagedProductDefinition/Enbrel055Package
Resource PackagedProductDefinition:
Profile: SubmittedMedicinalPackaging
MarketingStatuses
Status DateRange active 2020-03-05 --> (ongoing) packaging
identifier: National drug codes/58406-055-04
type: CARTON
Properties
Type Value[x] Combination Product Type Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) packaging
identifier: National drug codes/58406-055-01
type: SYRINGE
quantity: 4
containedItem
Items
Reference ManufacturedItemDefinition: status = active; manufacturedDoseForm = SOLUTION amount: 0.5 mL (Details: UCUM codemL = 'mL')
Entry 61 - fullUrl = http://example.org/RegulatedAuthorization/Enbrel055Marketing
Resource RegulatedAuthorization:
Profile: SubmittedMedicinalProductMarketing
identifier:
urn:oid:2.16.840.1.113883.3.150/NDA103795type: NDA
region: United States of America