FHIR for FAIR - FHIR Implementation Guide, published by Health Level Seven International - SOA Work Group. This guide is not an authorized publication; it is the continuous build for version 1.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/fhir-for-fair/ and changes regularly. See the Directory of published versions
The concept of FAIR digital object is quite wide and can vary in term of granularity and type of data that should be represented. (For a definition of a FAIR data object see section 4.1 of the EC publication Turning FAIR into reality and Wittenburg, P., Strawn, G., Mons, B. et al.: Digital objects as drivers towards convergence in data infrastructures 2019 - Full Text). It may be a single unit such as a coded diagnosis or a collection of machine actionable data (data set). It may represent a waveform, an image, a condition, other types of data or a combination of them.
This implies that their representation with HL7 FHIR resources and elements may range from a sinlge element to a set of linked resources. (see Figure 1).
Similar considerations can be done also for the metadata, metadata can be in fact represented by elements belonging to the same resource documenting the data and by a set of other linked resources.
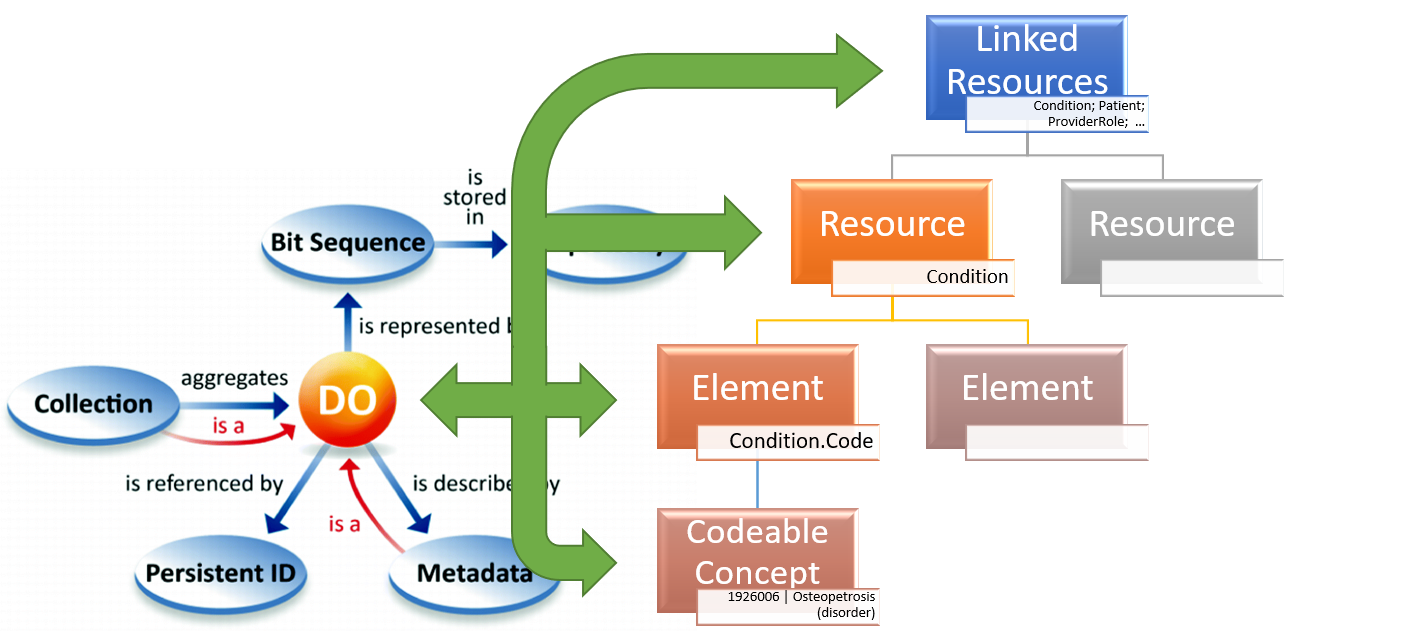
Figure 1 – FAIR Digital Object representation
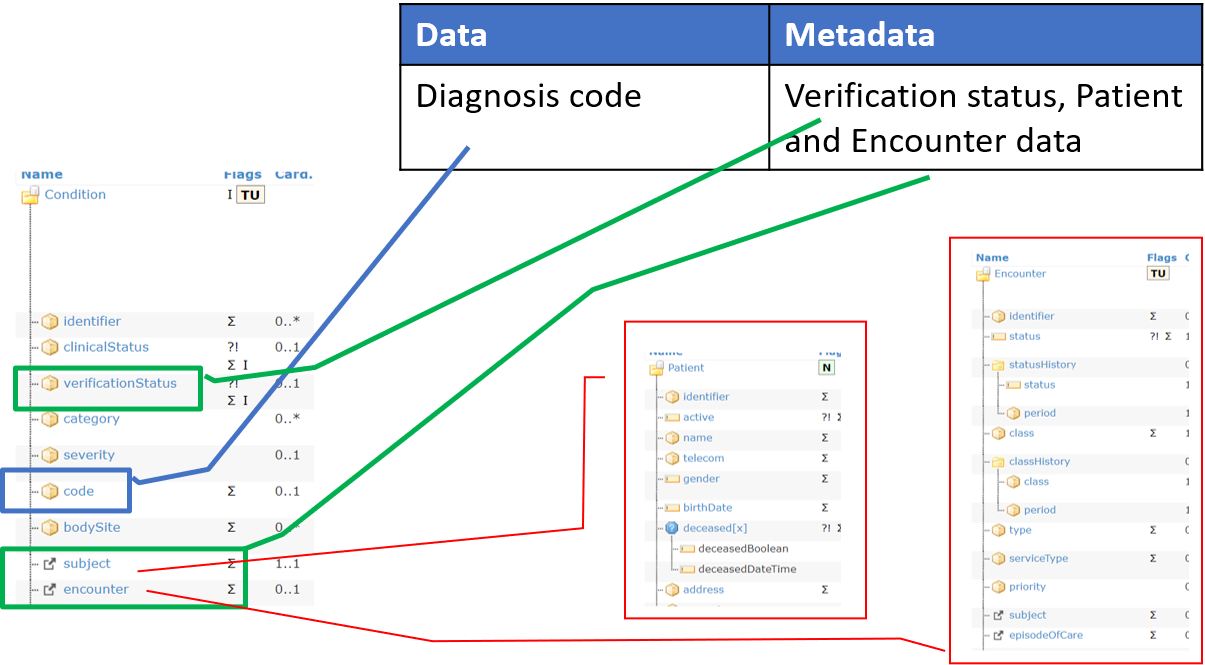
Figure 2 – Mapping FAIR data and metadata
The critical point is however the actual logical distinction between
metadata and data (both are FAIR DO); with metadata playing a key role
for making data findable, accessible, interoperable and reusable.
Metadata are in fact usually described as the “data about data”, but in
practice what is “data” and what is “metadata” is a matter of
perspective: based on the context, the same information can be in fact
considered as part of the data or of the metadata.
This makes even more complex the mapping of the FAIR DO to the FHIR resources.
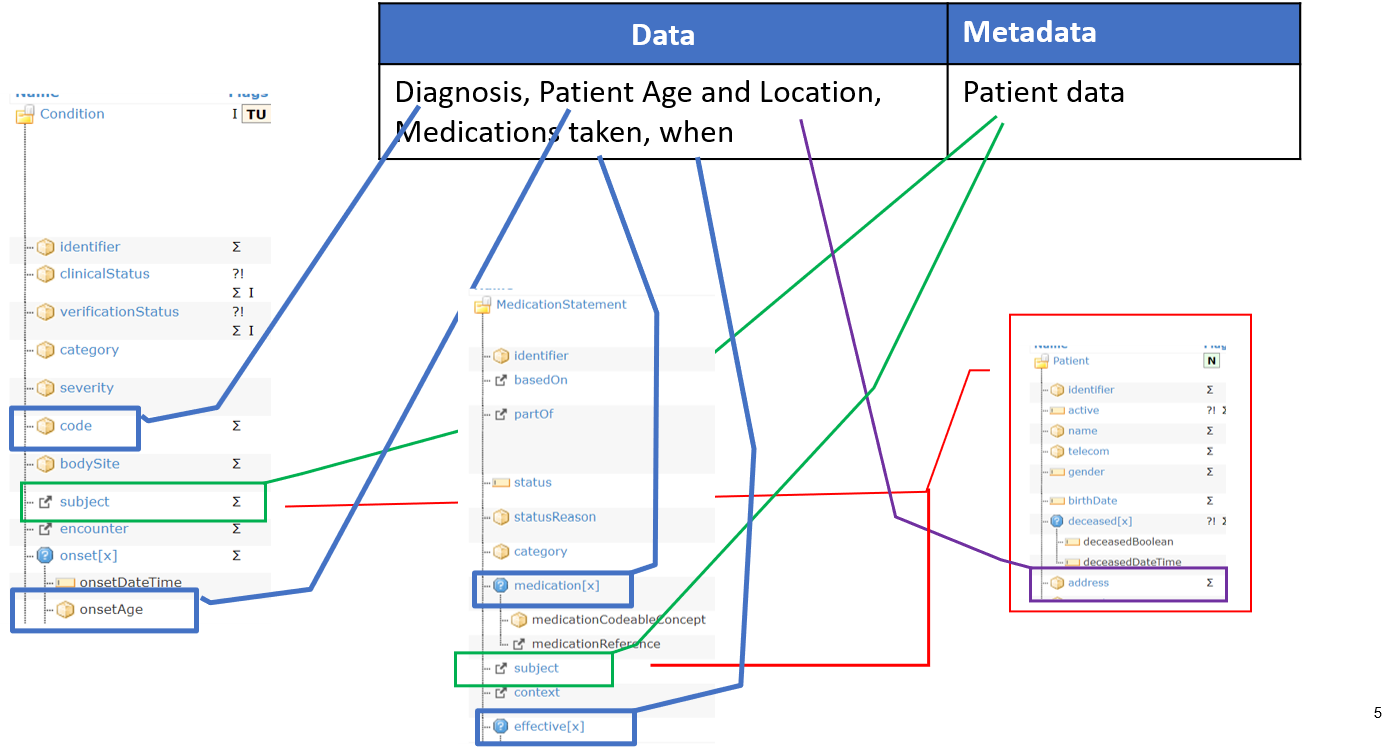
Figure 3 – Mapping FAIR data and metadata
In consideration of the previous arguments, the realization of the requirement for a metadata FAIR data object, distinct from the data FAIR data object, and identified by a persistent and unique ID need to be pragmatically evaluated, above all for FHIR implementations.
This means that depending on the context of use and on the granularity of data, distinct and identifiable FHIR resources can be used to record a selected set of metadata elements; but this might not be true for all the information that could be considered metadata.
Let’s consider for example the following levels:
Study Level: specific to a study, publication, or usage context (collection of subject level data).
Subject Level: specific to a single subject (patient).
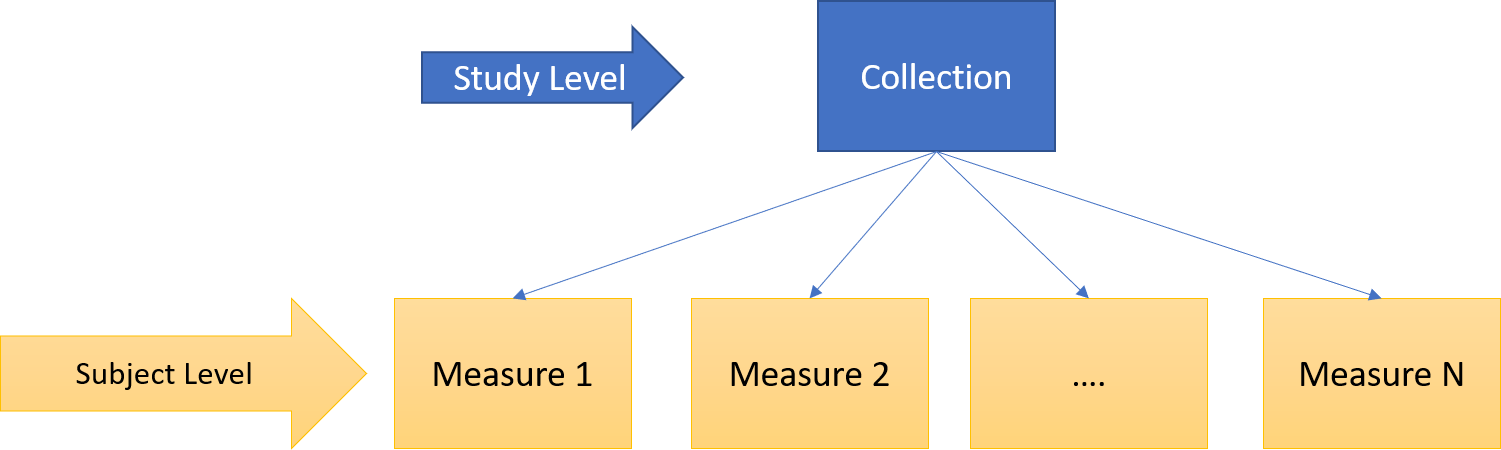
Figure 4 - Example of levels of data objects granularity
If for the study level we can assume data are represented by the subject level objects, distinct from the metadata describing the “study” (see figure below) ; this might be not so true for the subject level.
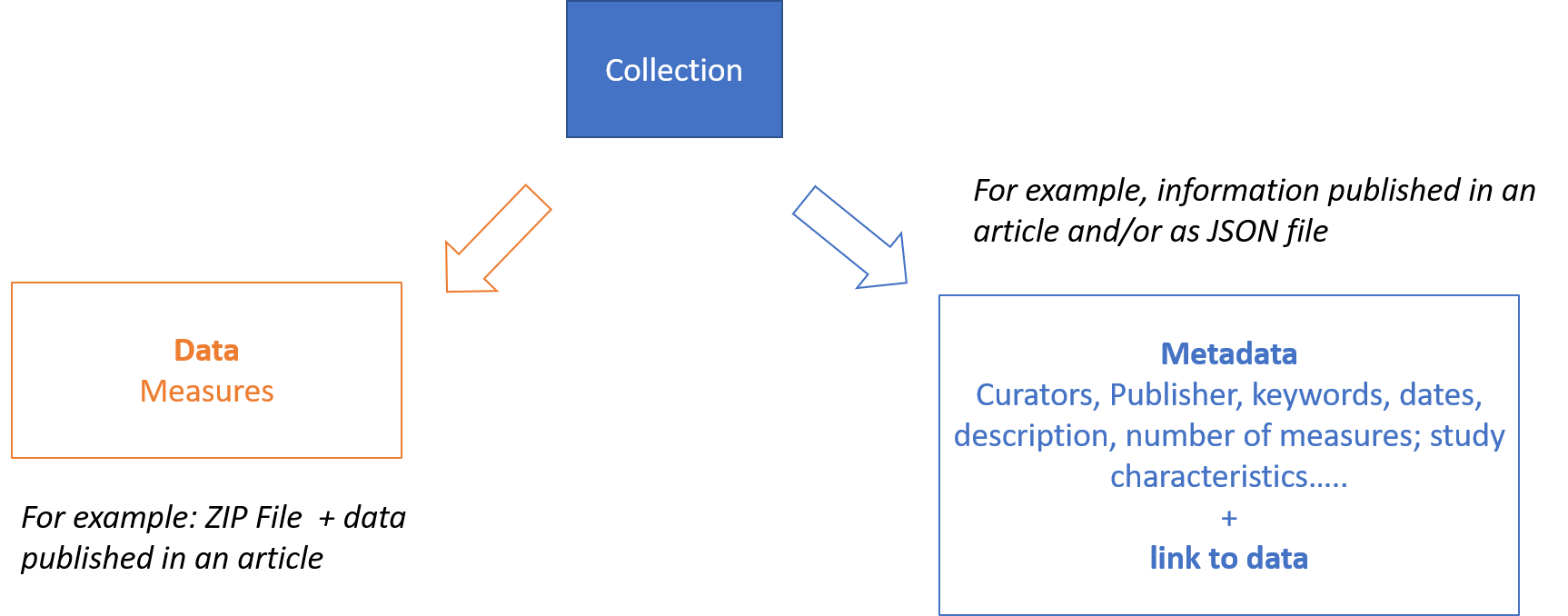
Figure 5 – Metadata and data for a data collection.
HL7 FHIR provides several candidates resources to represent metadata for collection of data (e.g., Bundle, Lists..) and the choice of the resource to be used strongly depend on the usage context. Library, Citation and ResearchStudy, seem to be the most promising resources to better support the “rich metadata” FAIR requirement; however not all of them are available in HL7 FHIR R4. Furthermore, since most of them have a low FHIR maturity level, they are subject to possible non-negligible changes, including the way the linkage between metadata (i.e. the resource itself) and data (i.e. the referred resources) is realized.
The kinds of data objects that the metadata may refer to can be:
Non-FHIR objects
FHIR and non-FHIR objects
FHIR-only objects
Depending on the “metadata” resource, the FHIR version chosen and the type of object to point to, different solutions can be adopted. For example, the current Library resource covers this requirement through the content element (Attachment datatype), in this case the link to data is recorded by using an uri (this may or may not be enough for referring FHIR resources). Citation (looking at the FHIR R5 specs) provides instead more flexible and comprehensive solutions through the citedArtifact.relatedTo.target[x] element; providing different means (uri, resource references, business identifiers) to handle these references.
It’s worth to mention that FHIR specifies a dedicated resources (Provenance) to record Provenance information typically part of the “metadata”.
As described above, at the subject level, the boundary between metadata and data is not always so sharp. In the example below derived from the Ninfea real world case is shown for example how the gestational week or the mother height and weight, might be considered metadata for the signals, but also patient level data.
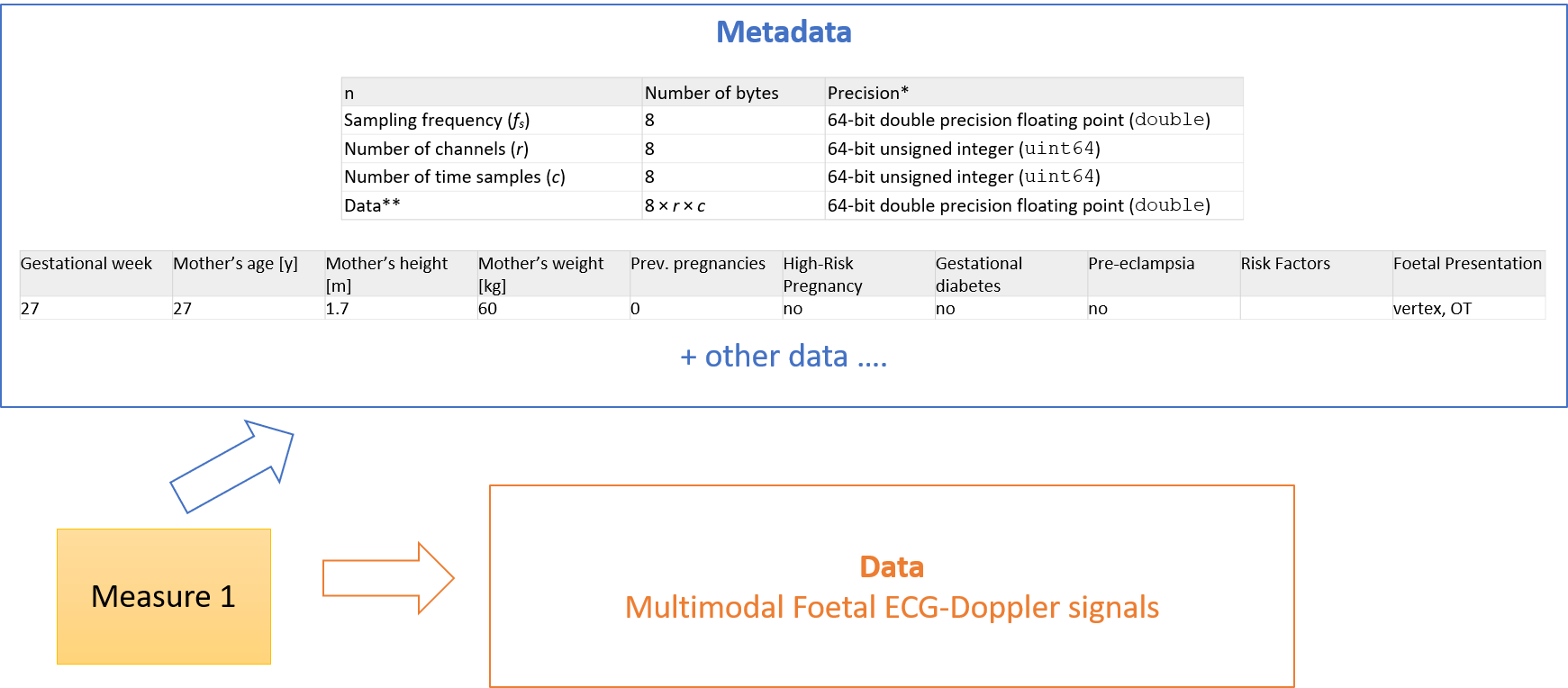
Figure 6 – Metadata and data at the subject level.
This guide will not therefore attempt to prescribe any tight separation of metadata/data at the subject level when FAIR is implemented by using HL7 FHIR.
There are HL7 FHIR resources (e.g. EvidenceReport, Bundle[collection], Composition, List; DocumentManifest) that can be potentially used to record some subject level metadata information as a distinct FHIR resource instance. Nevertheless, typically metadata information is recorded by a set of linked FHIR resources. For example, a Condition instance documenting a specific problem (the data), may use the subject, the encounter and the evidence references to Patient, Encounter and Observation FHIR instances to document the patient, encounter and other information used as evidence to describe the context of this problem (the metadata).
In case of non-FHIR data objects or mixed cases (FHIR and non-FHIR objects) implementers should evaluate the feasibility and the cost/benefit of transforming these objects (completely or partially) in FHIR instances; and/or creating FHIR resources (e.g., DocumentManifest) to document some metadata information.