 abstract
abstract baseDefinition
baseDefinition copyright
copyright date
date- Values Differ
 description
description experimental
experimental fhirVersion
fhirVersion jurisdiction
jurisdiction
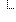 jurisdiction[0]
jurisdiction[0] kind
kind name
name publisher
publisher purpose
purpose status
status title
title type
type url
url version
version- Values Differ
| Left: | Ingredient - Drug Pq (http://hl7.org/fhir/uv/pharm-quality/StructureDefinition/Ingredient-drug-pq) |
| Right: | Ingredient - Drug Pq (http://hl7.org/fhir/uv/pharm-quality/StructureDefinition/Ingredient-drug-pq) |
| Error | StructureDefinition.version | Values for version differ: '1.0.0-ballot' vs '1.0.0' |
| Information | StructureDefinition.date | Values for date differ: '2023-12-18T16:00:45+00:00' vs '2024-05-08T12:53:57+00:00' |
| Information | StructureDefinition.short | Values for short differ: 'Additional content defined by implementations' vs 'Extension' |
| Information | StructureDefinition.definition | Values for definition differ: 'May be used to represent additional information that is not part of the basic definition of the resource. To make the use of extensions safe and managable, there is a strict set of governance applied to the definition and use of extensions. Though any implementer can define an extension, there is a set of requirements that SHALL be met as part of the definition of the extension.' vs 'An Extension' |
| Information | Ingredient.function | Example/preferred bindings differ at Ingredient.function using binding from IngredientDrugPq |
| Name | Value | Comments | |
|---|---|---|---|
 abstract abstract | false | ||
 baseDefinition baseDefinition | http://hl7.org/fhir/StructureDefinition/Ingredient | ||
 copyright copyright | |||
 date date | 2023-12-18T16:00:45+00:00 | 2024-05-08T12:53:57+00:00 |
|
 description description | This Ingredient profile represents the active ingredient(s) of a drug substance or product. It references one SubstanceDefinition resource and can include the strength or concentration of that substance. | ||
 experimental experimental | |||
 fhirVersion fhirVersion | 5.0.0 | ||
 jurisdiction jurisdiction | |||
 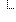 jurisdiction[0] jurisdiction[0] | http://unstats.un.org/unsd/methods/m49/m49.htm#001 | ||
 kind kind | resource | ||
 name name | IngredientDrugPq | ||
 publisher publisher | HL7 International / Biomedical Research and Regulation | ||
 purpose purpose | |||
 status status | active | ||
 title title | Ingredient - Drug Pq | ||
 type type | Ingredient | ||
 url url | http://hl7.org/fhir/uv/pharm-quality/StructureDefinition/Ingredient-drug-pq | ||
 version version | 1.0.0-ballot | 1.0.0 |
|
| Name | L Flags | L Card. | L Type | L Description & Constraints | R Flags | R Card. | R Type | R Description & Constraints | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | C | 0..* | Ingredient | An ingredient of a manufactured item or pharmaceutical product ing-1: If an ingredient is noted as an allergen (allergenicIndicator) then its substance should be a code. If the substance is a SubstanceDefinition, then the allegen information should be documented in that resource | C | 0..* | Ingredient | An ingredient of a manufactured item or pharmaceutical product ing-1: If an ingredient is noted as an allergen (allergenicIndicator) then its substance should be a code. If the substance is a SubstanceDefinition, then the allegen information should be documented in that resource | |||||||||
  | Σ | 0..1 | id | Logical id of this artifact | Σ | 0..1 | id | Logical id of this artifact | |||||||||
  | Σ | 0..1 | Meta | Metadata about the resource | Σ | 0..1 | Meta | Metadata about the resource | |||||||||
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created | ?!Σ | 0..1 | uri | A set of rules under which this content was created | |||||||||
  | 0..1 | code | Language of the resource content Binding: ?? (required): IETF language tag for a human language
| 0..1 | code | Language of the resource content Binding: ?? (required): IETF language tag for a human language
| |||||||||||
  | 0..1 | Narrative | Text summary of the resource, for human interpretation | 0..1 | Narrative | Text summary of the resource, for human interpretation | |||||||||||
  | 0..* | Resource | Contained, inline Resources | 0..* | Resource | Contained, inline Resources | |||||||||||
  | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Extension Slice: Unordered, Open by value:url | |||||||||||
  | ?!Σ | 0..* | Extension | Extensions that cannot be ignored | ?!Σ | 0..* | Extension | Extensions that cannot be ignored | |||||||||
  | SΣ | 0..1 | Identifier | An identifier or code by which the ingredient can be referenced | SΣ | 0..1 | Identifier | An identifier or code by which the ingredient can be referenced | |||||||||
  | ?!Σ | 1..1 | code | draft | active | retired | unknown Binding: ?? (required): The lifecycle status of an artifact. | ?!Σ | 1..1 | code | draft | active | retired | unknown Binding: ?? (required): The lifecycle status of an artifact. | |||||||||
  | Σ | 0..* | Reference(MedicinalProductDefinition | AdministrableProductDefinition | ManufacturedItemDefinition) | The product which this ingredient is a constituent part of | Σ | 0..* | Reference(MedicinalProductDefinition | AdministrableProductDefinition | ManufacturedItemDefinition) | The product which this ingredient is a constituent part of | |||||||||
  | Σ | 1..1 | CodeableConcept | Purpose of the ingredient within the product, e.g. active, inactive Binding: ?? (preferred): A classification of the ingredient identifying its purpose within the product, e.g. active, inactive. | Σ | 1..1 | CodeableConcept | Purpose of the ingredient within the product, e.g. active, inactive Binding: ?? (preferred): A classification of the ingredient identifying its purpose within the product, e.g. active, inactive. | |||||||||
   | 0..1 | id | Unique id for inter-element referencing |
| |||||||||||||
   | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url |
| |||||||||||||
   | Σ | 0..* | Coding | Code defined by a terminology system |
| ||||||||||||
   | Σ | 0..1 | string | Plain text representation of the concept |
| ||||||||||||
  | Σ | 0..* | CodeableConcept | Precise action within the drug product, e.g. antioxidant, alkalizing agent Binding: ?? (preferred): A classification of the ingredient identifying its precise purpose(s) in the drug product. This extends the Ingredient.role to add more detail. Example: antioxidant, alkalizing agent. | Σ | 0..* | CodeableConcept | Precise action within the drug product, e.g. antioxidant, alkalizing agent Binding: ?? (example): A classification of the ingredient identifying its precise purpose(s) in the drug product. This extends the Ingredient.role to add more detail. Example: antioxidant, alkalizing agent. |
| ||||||||
  | Σ | 0..1 | CodeableConcept | A classification of the ingredient according to where in the physical item it tends to be used, such the outer shell of a tablet, inner body or ink | Σ | 0..1 | CodeableConcept | A classification of the ingredient according to where in the physical item it tends to be used, such the outer shell of a tablet, inner body or ink | |||||||||
  | ΣC | 0..1 | boolean | If the ingredient is a known or suspected allergen | ΣC | 0..1 | boolean | If the ingredient is a known or suspected allergen | |||||||||
  | 0..1 | markdown | A place for providing any notes that are relevant to the component, e.g. removed during process, adjusted for loss on drying | 0..1 | markdown | A place for providing any notes that are relevant to the component, e.g. removed during process, adjusted for loss on drying | |||||||||||
  | Σ | 0..* | BackboneElement | An organization that manufactures this ingredient | Σ | 0..* | BackboneElement | An organization that manufactures this ingredient | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 0..1 | code | allowed | possible | actual Binding: ?? (required): The way in which this manufacturer is associated with the ingredient. | Σ | 0..1 | code | allowed | possible | actual Binding: ?? (required): The way in which this manufacturer is associated with the ingredient. | |||||||||
   | Σ | 1..1 | Reference(Organization) | An organization that manufactures this ingredient | Σ | 1..1 | Reference(Organization) | An organization that manufactures this ingredient | |||||||||
  | ΣC | 1..1 | BackboneElement | The substance that comprises this ingredient | ΣC | 1..1 | BackboneElement | The substance that comprises this ingredient | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | SΣC | 1..1 | CodeableReference(SubstanceDefinition - Component Substance Drug PQ) | Ingredient Substance Binding: ?? (example): This value set includes all substance codes from SNOMED CT - provided as an exemplar value set. | SΣC | 1..1 | CodeableReference(SubstanceDefinition - Component Substance Drug PQ) | Ingredient Substance Binding: ?? (example): This value set includes all substance codes from SNOMED CT - provided as an exemplar value set. | |||||||||
   | Σ | 0..* | BackboneElement | The quantity of substance, per presentation, or per volume or mass, and type of quantity | Σ | 0..* | BackboneElement | The quantity of substance, per presentation, or per volume or mass, and type of quantity | |||||||||
    | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
    | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
    | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
    | Σ | 0..1 | Ratio, RatioRange, CodeableConcept, Quantity | The quantity of substance in the unit of presentation | Σ | 0..1 | Ratio, RatioRange, CodeableConcept, Quantity | The quantity of substance in the unit of presentation | |||||||||
    | Σ | 0..1 | string | Text of either the whole presentation strength or a part of it (rest being in Strength.presentation as a ratio) | Σ | 0..1 | string | Text of either the whole presentation strength or a part of it (rest being in Strength.presentation as a ratio) | |||||||||
    | Σ | 0..1 | Ratio, RatioRange, CodeableConcept, Quantity | The strength per unitary volume (or mass) | Σ | 0..1 | Ratio, RatioRange, CodeableConcept, Quantity | The strength per unitary volume (or mass) | |||||||||
    | Σ | 0..1 | string | Text of either the whole concentration strength or a part of it (rest being in Strength.concentration as a ratio) | Σ | 0..1 | string | Text of either the whole concentration strength or a part of it (rest being in Strength.concentration as a ratio) | |||||||||
    | Σ | 0..1 | CodeableConcept | A code that indicates if the strength is, for example, based on the ingredient substance as stated or on the substance base (when the ingredient is a salt) | Σ | 0..1 | CodeableConcept | A code that indicates if the strength is, for example, based on the ingredient substance as stated or on the substance base (when the ingredient is a salt) | |||||||||
    | Σ | 0..1 | string | When strength is measured at a particular point or distance | Σ | 0..1 | string | When strength is measured at a particular point or distance | |||||||||
    | Σ | 0..* | CodeableConcept | Where the strength range applies Binding: ?? (example): Jurisdiction codes | Σ | 0..* | CodeableConcept | Where the strength range applies Binding: ?? (example): Jurisdiction codes | |||||||||
    | SΣ | 0..* | BackboneElement | Strength expressed in terms of a reference substance | SΣ | 0..* | BackboneElement | Strength expressed in terms of a reference substance | |||||||||
     | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
     | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
     | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
     | Σ | 1..1 | CodeableReference(SubstanceDefinition) | Relevant reference substance Binding: ?? (example): This value set includes all substance codes from SNOMED CT - provided as an exemplar value set. | Σ | 1..1 | CodeableReference(SubstanceDefinition) | Relevant reference substance Binding: ?? (example): This value set includes all substance codes from SNOMED CT - provided as an exemplar value set. | |||||||||
     | Σ | 1..1 | Ratio, RatioRange, Quantity | Strength expressed in terms of a reference substance | Σ | 1..1 | Ratio, RatioRange, Quantity | Strength expressed in terms of a reference substance | |||||||||
     | Σ | 0..1 | string | When strength is measured at a particular point or distance | Σ | 0..1 | string | When strength is measured at a particular point or distance | |||||||||
     | Σ | 0..* | CodeableConcept | Where the strength range applies Binding: ?? (example): Jurisdiction codes | Σ | 0..* | CodeableConcept | Where the strength range applies Binding: ?? (example): Jurisdiction codes | |||||||||
 Documentation for this format Documentation for this format | |||||||||||||||||