 abstract
abstract baseDefinition
baseDefinition copyright
copyright date
date- Values Differ
 description
description experimental
experimental fhirVersion
fhirVersion jurisdiction
jurisdiction
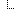 jurisdiction[0]
jurisdiction[0] kind
kind name
name publisher
publisher purpose
purpose status
status title
title type
type url
url version
version- Values Differ
| Left: | Adverse Event Clinical Research (http://hl7.org/fhir/uv/ae-research-ig/StructureDefinition/AdverseEvent-clinical-research) |
| Right: | Adverse Event Clinical Research (http://hl7.org/fhir/uv/ae-research-ig/StructureDefinition/AdverseEvent-clinical-research) |
| Error | StructureDefinition.version | Values for version differ: '1.0.0' vs '1.0.1' |
| Information | StructureDefinition.date | Values for date differ: '2024-04-15T17:39:57+00:00' vs '2024-04-30T19:42:09+00:00' |
| Name | Value | Comments | |
|---|---|---|---|
 abstract abstract | false | ||
 baseDefinition baseDefinition | http://hl7.org/fhir/StructureDefinition/AdverseEvent | ||
 copyright copyright | |||
 date date | 2024-04-15T17:39:57+00:00 | 2024-04-30T19:42:09+00:00 |
|
 description description | Foundational profile of AdverseEvent for Clinical Research communications. | ||
 experimental experimental | |||
 fhirVersion fhirVersion | 5.0.0 | ||
 jurisdiction jurisdiction | |||
 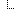 jurisdiction[0] jurisdiction[0] | http://unstats.un.org/unsd/methods/m49/m49.htm#001 | ||
 kind kind | resource | ||
 name name | AdverseEventClinicalResearch | ||
 publisher publisher | HL7 International / Biomedical Research and Regulation | ||
 purpose purpose | |||
 status status | active | ||
 title title | Adverse Event Clinical Research | ||
 type type | AdverseEvent | ||
 url url | http://hl7.org/fhir/uv/ae-research-ig/StructureDefinition/AdverseEvent-clinical-research | ||
 version version | 1.0.0 | 1.0.1 |
|
| Name | L Flags | L Card. | L Type | L Description & Constraints | R Flags | R Card. | R Type | R Description & Constraints | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | C | 0..* | AdverseEvent | An event that may be related to unintended effects on a patient or research participant aeClinRes-seriousness-1: If seriousness is serious then must have at least one seriousness criterion. | C | 0..* | AdverseEvent | An event that may be related to unintended effects on a patient or research participant aeClinRes-seriousness-1: If seriousness is serious then must have at least one seriousness criterion. | |||||||||
  | Σ | 0..1 | id | Logical id of this artifact | Σ | 0..1 | id | Logical id of this artifact | |||||||||
  | Σ | 0..1 | Meta | Metadata about the resource | Σ | 0..1 | Meta | Metadata about the resource | |||||||||
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created | ?!Σ | 0..1 | uri | A set of rules under which this content was created | |||||||||
  | 0..1 | code | Language of the resource content Binding: ?? (required): IETF language tag for a human language
| 0..1 | code | Language of the resource content Binding: ?? (required): IETF language tag for a human language
| |||||||||||
  | 0..1 | Narrative | Text summary of the resource, for human interpretation | 0..1 | Narrative | Text summary of the resource, for human interpretation | |||||||||||
  | 0..* | Resource | Contained, inline Resources | 0..* | Resource | Contained, inline Resources | |||||||||||
  | 0..* | Extension | Extension Slice: Unordered, Open by value:url | 0..* | Extension | Extension Slice: Unordered, Open by value:url | |||||||||||
  | ?!Σ | 0..* | Extension | Extensions that cannot be ignored | ?!Σ | 0..* | Extension | Extensions that cannot be ignored | |||||||||
  | Σ | 0..* | Identifier | Business identifier for the event | Σ | 0..* | Identifier | Business identifier for the event | |||||||||
  | ?!Σ | 1..1 | code | in-progress | completed | entered-in-error | unknown Binding: ?? (required): Codes identifying the lifecycle stage of an event. | ?!Σ | 1..1 | code | in-progress | completed | entered-in-error | unknown Binding: ?? (required): Codes identifying the lifecycle stage of an event. | |||||||||
  | ?!Σ | 1..1 | code | actual Binding: ?? (required): Overall nature of the adverse event, e.g. real or potential. Required Pattern: actual | ?!Σ | 1..1 | code | actual Binding: ?? (required): Overall nature of the adverse event, e.g. real or potential. Required Pattern: actual | |||||||||
  | Σ | 0..* | CodeableConcept | wrong-patient | procedure-mishap | medication-mishap | device | unsafe-physical-environment | hospital-aquired-infection | wrong-body-site Binding: ?? (example): Overall categorization of the event, e.g. product-related or situational. | Σ | 0..* | CodeableConcept | wrong-patient | procedure-mishap | medication-mishap | device | unsafe-physical-environment | hospital-aquired-infection | wrong-body-site Binding: ?? (example): Overall categorization of the event, e.g. product-related or situational. | |||||||||
  | Σ | 1..1 | CodeableConcept | Event or incident that occurred or was averted Binding: ?? (example): Detailed type of event. | Σ | 1..1 | CodeableConcept | Event or incident that occurred or was averted Binding: ?? (example): Detailed type of event. | |||||||||
  | Σ | 1..1 | Reference(Patient | Group | Practitioner | RelatedPerson | ResearchSubject) | Subject impacted by event | Σ | 1..1 | Reference(Patient | Group | Practitioner | RelatedPerson | ResearchSubject) | Subject impacted by event | |||||||||
  | Σ | 0..1 | Reference(Encounter) | The Encounter associated with the start of the AdverseEvent | Σ | 0..1 | Reference(Encounter) | The Encounter associated with the start of the AdverseEvent | |||||||||
  | SΣ | 0..1 | Period | When the event occurred | SΣ | 0..1 | Period | When the event occurred | |||||||||
  | Σ | 0..1 | dateTime | When the event was detected | Σ | 0..1 | dateTime | When the event was detected | |||||||||
  | Σ | 0..1 | dateTime | When the event was recorded | Σ | 0..1 | dateTime | When the event was recorded | |||||||||
  | Σ | 0..* | Reference(Condition | Observation) | Effect on the subject due to this event | Σ | 0..* | Reference(Condition | Observation) | Effect on the subject due to this event | |||||||||
  | Σ | 0..1 | Reference(Location) | Location where adverse event occurred | Σ | 0..1 | Reference(Location) | Location where adverse event occurred | |||||||||
  | SΣ | 1..1 | CodeableConcept | Investigator defined severity of the adverse event, in relation to the subject not the resulting condition Binding: ?? (required) | SΣ | 1..1 | CodeableConcept | Investigator defined severity of the adverse event, in relation to the subject not the resulting condition Binding: ?? (required) | |||||||||
  | SΣ | 1..1 | CodeableConcept | Type of outcome from the adverse event Binding: ?? (required) | SΣ | 1..1 | CodeableConcept | Type of outcome from the adverse event Binding: ?? (required) | |||||||||
  | Σ | 0..1 | Reference(Patient | Practitioner | PractitionerRole | RelatedPerson | ResearchSubject) | Who recorded the adverse event | Σ | 0..1 | Reference(Patient | Practitioner | PractitionerRole | RelatedPerson | ResearchSubject) | Who recorded the adverse event | |||||||||
  | Σ | 0..* | BackboneElement | Who was involved in the adverse event or the potential adverse event and what they did | Σ | 0..* | BackboneElement | Who was involved in the adverse event or the potential adverse event and what they did | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 0..1 | CodeableConcept | Type of involvement Binding: ?? (example): Codes describing the type of involvement of the actor in the adverse event. | Σ | 0..1 | CodeableConcept | Type of involvement Binding: ?? (example): Codes describing the type of involvement of the actor in the adverse event. | |||||||||
   | Σ | 1..1 | Reference(Practitioner | PractitionerRole | Organization | CareTeam | Patient | Device | RelatedPerson | ResearchSubject) | Who was involved in the adverse event or the potential adverse event | Σ | 1..1 | Reference(Practitioner | PractitionerRole | Organization | CareTeam | Patient | Device | RelatedPerson | ResearchSubject) | Who was involved in the adverse event or the potential adverse event | |||||||||
  | SΣ | 1..1 | Reference(ResearchStudy) | Research study that the subject is enrolled in | SΣ | 1..1 | Reference(ResearchStudy) | Research study that the subject is enrolled in | |||||||||
  | 0..1 | boolean | Considered likely or probable or anticipated in the research study | 0..1 | boolean | Considered likely or probable or anticipated in the research study | |||||||||||
  | Σ | 0..* | BackboneElement | The suspected agent causing the adverse event | Σ | 0..* | BackboneElement | The suspected agent causing the adverse event | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 1..1 | CodeableConcept, Reference(Immunization | Procedure | Substance | Medication | MedicationAdministration | MedicationStatement | Device | BiologicallyDerivedProduct | ResearchStudy) | Refers to the specific entity that caused the adverse event | Σ | 1..1 | CodeableConcept, Reference(Immunization | Procedure | Substance | Medication | MedicationAdministration | MedicationStatement | Device | BiologicallyDerivedProduct | ResearchStudy) | Refers to the specific entity that caused the adverse event | |||||||||
   | SΣ | 1..1 | BackboneElement | Information on the possible cause of the event | SΣ | 1..1 | BackboneElement | Information on the possible cause of the event | |||||||||
    | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
    | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
    | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
    | Σ | 0..1 | CodeableConcept | Method of evaluating the relatedness of the suspected entity to the event. Binding: ?? (preferred): Codes for the method of evaluating the relatedness of the suspected entity to the event, such as ProbabilityScale | Bayesian | Checklist. | Σ | 0..1 | CodeableConcept | Method of evaluating the relatedness of the suspected entity to the event. Binding: ?? (preferred): Codes for the method of evaluating the relatedness of the suspected entity to the event, such as ProbabilityScale | Bayesian | Checklist. | |||||||||
    | Σ | 0..1 | CodeableConcept | Result of the assessment regarding the relatedness of the suspected entity to the event Binding: ?? (preferred) | Σ | 0..1 | CodeableConcept | Result of the assessment regarding the relatedness of the suspected entity to the event Binding: ?? (preferred) | |||||||||
    | Σ | 0..1 | Reference(Practitioner | PractitionerRole | Patient | RelatedPerson | ResearchSubject) | Author of the information on the possible cause of the event | Σ | 0..1 | Reference(Practitioner | PractitionerRole | Patient | RelatedPerson | ResearchSubject) | Author of the information on the possible cause of the event | |||||||||
  | Σ | 0..* | BackboneElement | Contributing factors suspected to have increased the probability or severity of the adverse event | Σ | 0..* | BackboneElement | Contributing factors suspected to have increased the probability or severity of the adverse event | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 1..1 | Reference(Condition | Observation | AllergyIntolerance | FamilyMemberHistory | Immunization | Procedure | Device | DeviceUsage | DocumentReference | MedicationAdministration | MedicationStatement), CodeableConcept | Item suspected to have increased the probability or severity of the adverse event Binding: ?? (example): Codes describing the contributing factors suspected to have increased the probability or severity of the adverse event. | Σ | 1..1 | Reference(Condition | Observation | AllergyIntolerance | FamilyMemberHistory | Immunization | Procedure | Device | DeviceUsage | DocumentReference | MedicationAdministration | MedicationStatement), CodeableConcept | Item suspected to have increased the probability or severity of the adverse event Binding: ?? (example): Codes describing the contributing factors suspected to have increased the probability or severity of the adverse event. | |||||||||
  | Σ | 0..0 | Σ | 0..0 | |||||||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 1..1 | Reference(Immunization | Procedure | DocumentReference | MedicationAdministration | MedicationRequest), CodeableConcept | Action that contributed to avoiding the adverse event Binding: ?? (example): Codes describing the preventive actions that contributed to avoiding the adverse event. | Σ | 1..1 | Reference(Immunization | Procedure | DocumentReference | MedicationAdministration | MedicationRequest), CodeableConcept | Action that contributed to avoiding the adverse event Binding: ?? (example): Codes describing the preventive actions that contributed to avoiding the adverse event. | |||||||||
  | Σ | 0..* | BackboneElement | Ameliorating actions taken after the adverse event occured in order to reduce the extent of harm | Σ | 0..* | BackboneElement | Ameliorating actions taken after the adverse event occured in order to reduce the extent of harm | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 1..1 | Reference(Procedure | DocumentReference | MedicationAdministration | MedicationRequest), CodeableConcept | Ameliorating action taken after the adverse event occured in order to reduce the extent of harm Binding: ?? (example): Codes describing the ameliorating actions taken after the adverse event occured in order to reduce the extent of harm. | Σ | 1..1 | Reference(Procedure | DocumentReference | MedicationAdministration | MedicationRequest), CodeableConcept | Ameliorating action taken after the adverse event occured in order to reduce the extent of harm Binding: ?? (example): Codes describing the ameliorating actions taken after the adverse event occured in order to reduce the extent of harm. | |||||||||
  | Σ | 0..* | BackboneElement | Supporting information relevant to the event | Σ | 0..* | BackboneElement | Supporting information relevant to the event | |||||||||
   | 0..1 | string | Unique id for inter-element referencing | 0..1 | string | Unique id for inter-element referencing | |||||||||||
   | 0..* | Extension | Additional content defined by implementations | 0..* | Extension | Additional content defined by implementations | |||||||||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | |||||||||
   | Σ | 1..1 | Reference(Condition | Observation | AllergyIntolerance | FamilyMemberHistory | Immunization | Procedure | DocumentReference | MedicationAdministration | MedicationStatement | QuestionnaireResponse), CodeableConcept | Subject medical history or document relevant to this adverse event Binding: ?? (example): Codes describing the supporting information relevant to the event. | Σ | 1..1 | Reference(Condition | Observation | AllergyIntolerance | FamilyMemberHistory | Immunization | Procedure | DocumentReference | MedicationAdministration | MedicationStatement | QuestionnaireResponse), CodeableConcept | Subject medical history or document relevant to this adverse event Binding: ?? (example): Codes describing the supporting information relevant to the event. | |||||||||
  | Σ | 0..* | Annotation | Comment on adverse event | Σ | 0..* | Annotation | Comment on adverse event | |||||||||
 Documentation for this format Documentation for this format | |||||||||||||||||