Clinical Practice Guidelines, published by HL7 International / Clinical Decision Support. This guide is not an authorized publication; it is the continuous build for version 2.0.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/cqf-recommendations/ and changes regularly. See the Directory of published versions
HIV Screening Guideline Recommendations
Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings
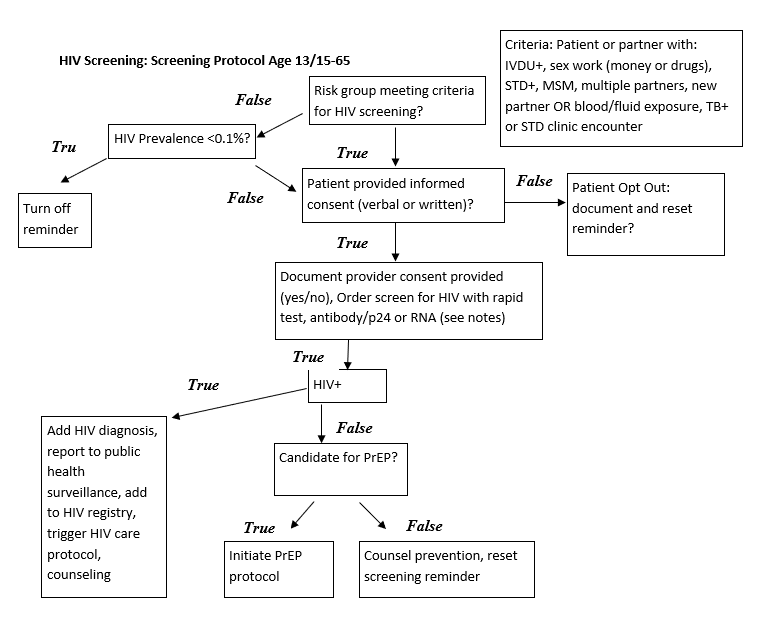
Recommends screening all individuals 13-65, including all pregnant women presenting from initial diagnosis through those presenting in labor. Recommends more frequent screening for individuals at high risk.
Supplemental Content USPTF June 2019 HIV Screening
Screening for HIV Infection - US Preventive Services Task Force Recommendation Statement
Recommends screening all individuals 15-65, including all pregnant women presenting from initial diagnosis through those presenting in labor. Recommends screening for younger or older individuals who are determined to be at high risk. Recommends more frequent screening for individuals at high risk.
Factors Conferring "High Risk" Category Membership
- Male-to-male sexual contact
- IV drug use
- Heterosexual or other contact with:
- Anal intercourse without condom
- Vaginal intercourse without a condom and with more than 1 partner whose HIV status is unknown
- Individuals who exchange sex for drugs or money
- Having STI
- Partner with STI
- Partner with HIV
- Partner in other high-risk category above
- People requesting STI testing
- Patients with TB
- Healthcare exposure to blood or other infectious bodily fluid
- Request for pre-exposure screening prior to new partner
Screening in pregnancy:
Screening for HIV Infection in Pregnant Women
- All women should be screened for HIV as part of early or initial prenatal screening
- Women in high risk category or in area with high prevalence of HIV should be rescreened in the third trimester
Consent:
- Patient will be notified that testing is being ordered either in person verbally or in writing
- Patient may opt out of testing at this time—if patient opts out this should be documented
- Patient does not require counseling to be tested
- Educational material should be offered to patient in appropriate health literacy level, modality and language
Testing:
Pregnant women in labor, recommend Rapid HIV test—otherwise routine HIV testing
- Rapid testing: Rapid HIV test IF POSITIVE then:
- also order HIV-1 and HIV-2 with HIV-1 p24 antigen IF POSITIVE then use routine testing pathway
- IF NEGATIVE:
- OR
- Routine testing: HIV-1 and HIV-2 antibodies with HIV-1 p24 antigen with reflex (automatic) supplemental testing if positive
- IF NEGATIVE: STOP
- AND: If supplemental testing indeterminate:
-
HIV-1 RNA
- After all NEGATIVE testing:
- Routine safe sex counseling
- After all POSITIVE testing:
- Optimally there would be an HIV treatment CDS pathway triggered
- At a minimum:
- Counseling
- Referral for HIV specialty treatment
- HIV viral load test
Rescreening:
- All individuals should have at least one lifetime HIV test
- Individuals at high risk should be screened at LEAST annually:
- Selected individuals at highest risk should be screened more frequently up to every 3-6 months
- Individuals seeking STI testing should be screened at every visit
Exceptions to screening* (2006 guideline only):
- If prevalence in practice is <0.1%, routine screening can be omitted in patients who do not have any high risk factors and who are not pregnant
HIV Screening Protocol