2022 CDC Clinical Practice Guideline for Prescribing Opioids Implementation Guide
2022.1.0 - CI Build
2022 CDC Clinical Practice Guideline for Prescribing Opioids Implementation Guide, published by Centers for Disease Control and Prevention (CDC). This guide is not an authorized publication; it is the continuous build for version 2022.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/opioid-cds-r4/ and changes regularly. See the Directory of published versions
Recommendations #7 (2022 CDC Clinical Practice Guideline for Prescribing Opioids for Pain):
Clinicians should evaluate benefits and risks with patients within 1-4 weeks of starting opioid therapy for subacute or chronic pain or of dosage escalation. Clinicians should regularly reevaluate benefits and risks of continued opioid therapy with patients (recommendation category: A; evidence type: 4).
Contents
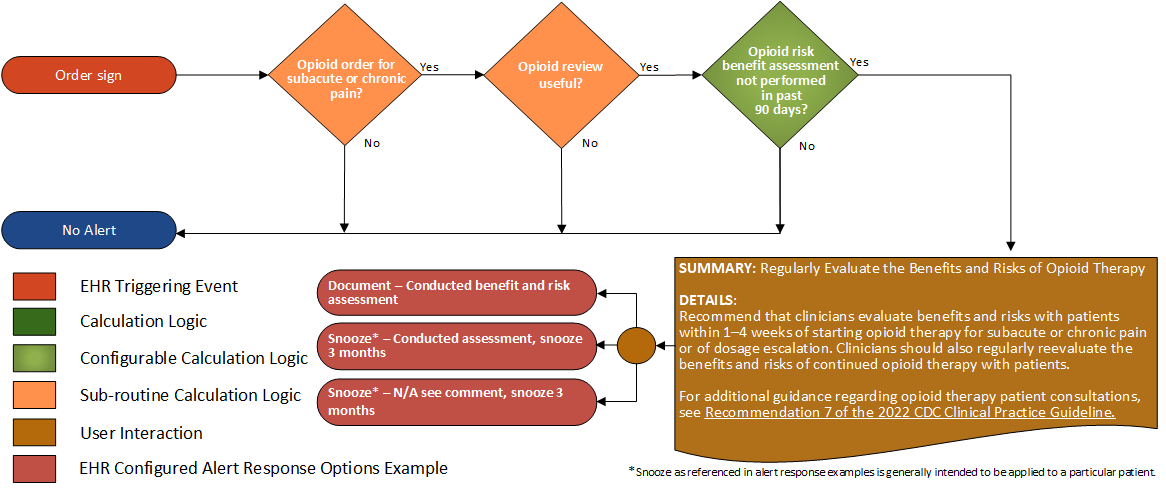
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Opioid order for subacute or chronic pain? | Yes | See For Subacute or Chronic Pain sub-routine | |||
| Opioid review useful? | Yes | See Opioid Review Useful sub-routine | |||
| Opioid risk - benefit assessment procedure not performed in last 90 days? | Yes | Absence of an opioid treatment assessment procedure in the last 90 days | Opioid treatment assessment procedure | Procedure | Procedure.code, and Procedure.performed |
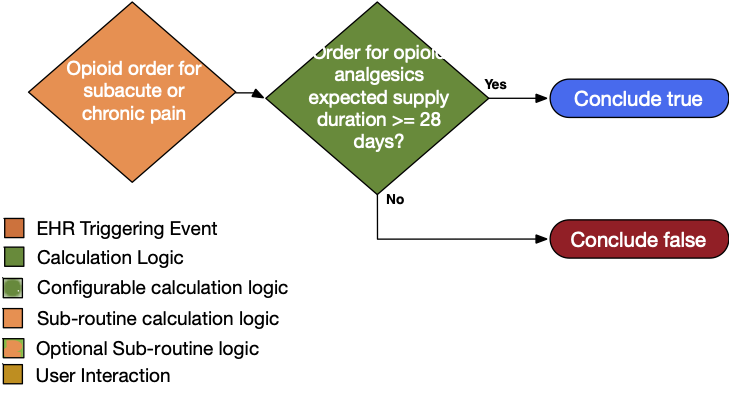
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Order for opioid analgesic with expected supply duration ≥ 28 days | Yes | Order for opioid analgesics with ambulatory misuse potential with a supply duration ≥ 28 days • Subacute definition = order for opioid analgesic with ambulatory misuse potential with a supply duration of one to two months • Chronic pain definition = order for opioid analgesic with ambulatory misuse potential with a supply duration of ≥ two months |
Opioid analgesics with ambulatory misuse potential | MedicationRequest | MedicationRequest.medication and MedicationRequest.dispenseRequest.expectedSupplyDuration |
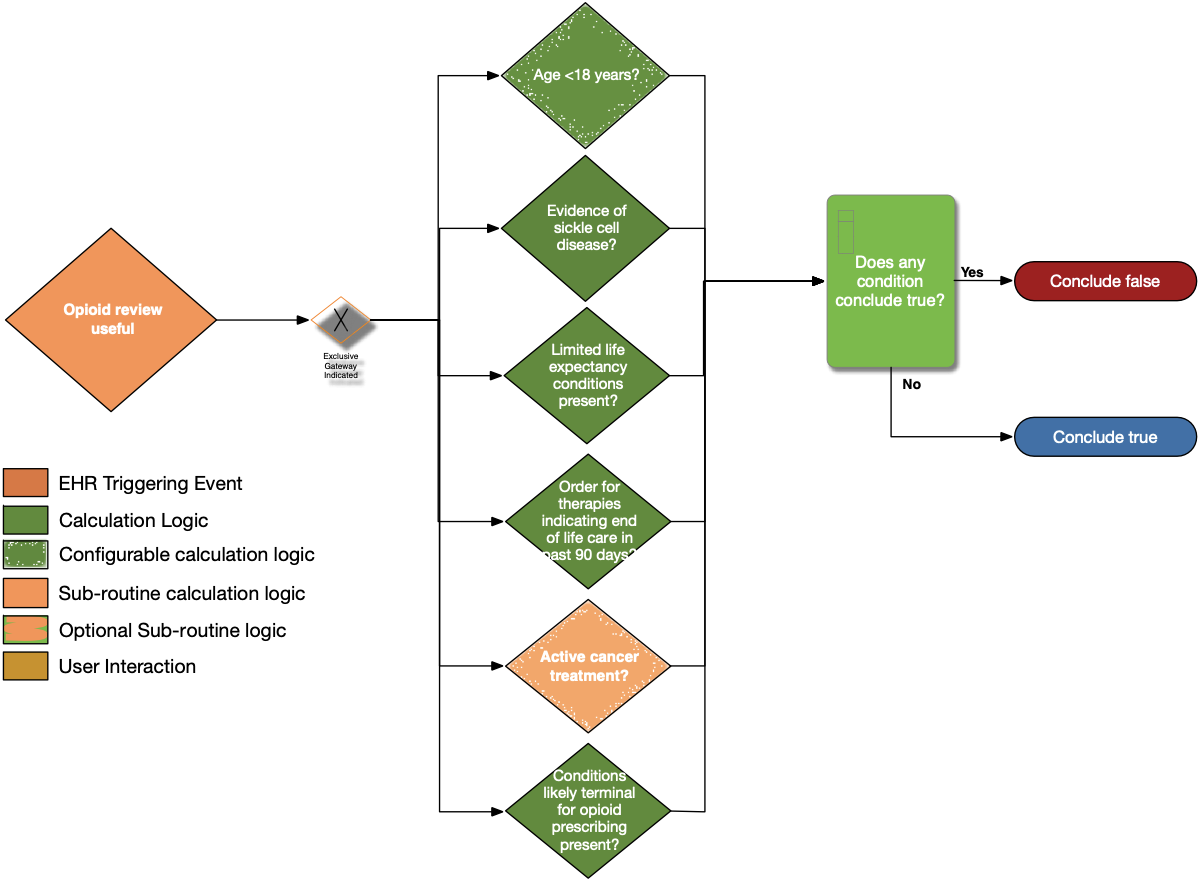
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Age < 18 years? | No | Calculate age from date of birth; exclude patients with age less than 18 years at the time of the prescription | Date of birth | Patient | Patient.birthDate |
| Evidence of sickle cell disease? | No | Look for patients with a diagnosis or problem list entry indicating sickle cell disease | Sickle cell disease condition | Condition | Condition.category, Condition.code, and Condition.clinicalStatus |
| Limited life expectancy conditions present? | No | Look for documented findings consistent with those listed in the limited life expectancy value set (terminal illness, bad prognosis, pre-terminal) | Limited life expectancy conditions | Condition | Condition.category and Condition.code |
| Order for therapies indicating end of life care in past 90 days? | No | Look for patients with an existing order for therapies indicating end of life care written within past 90 days | Therapies indicating end of life care | ServiceRequest | ServiceRequest.status, ServiceRequest.intent, ServiceRequest.authoredOn, and ServiceRequest.code |
| Active cancer treatment? | No | See Active Cancer Treatment sub-routine | See Active Cancer Treatment sub-routine | ||
| Conditions Likely Terminal for opioid prescribing present? | No | Look for patients with active conditions in the value set end-of-life-conditions | Conditions likely terminal for opioid prescribing | Condition | Condition.category, Condition.code, and Condition.clinicalStatus |
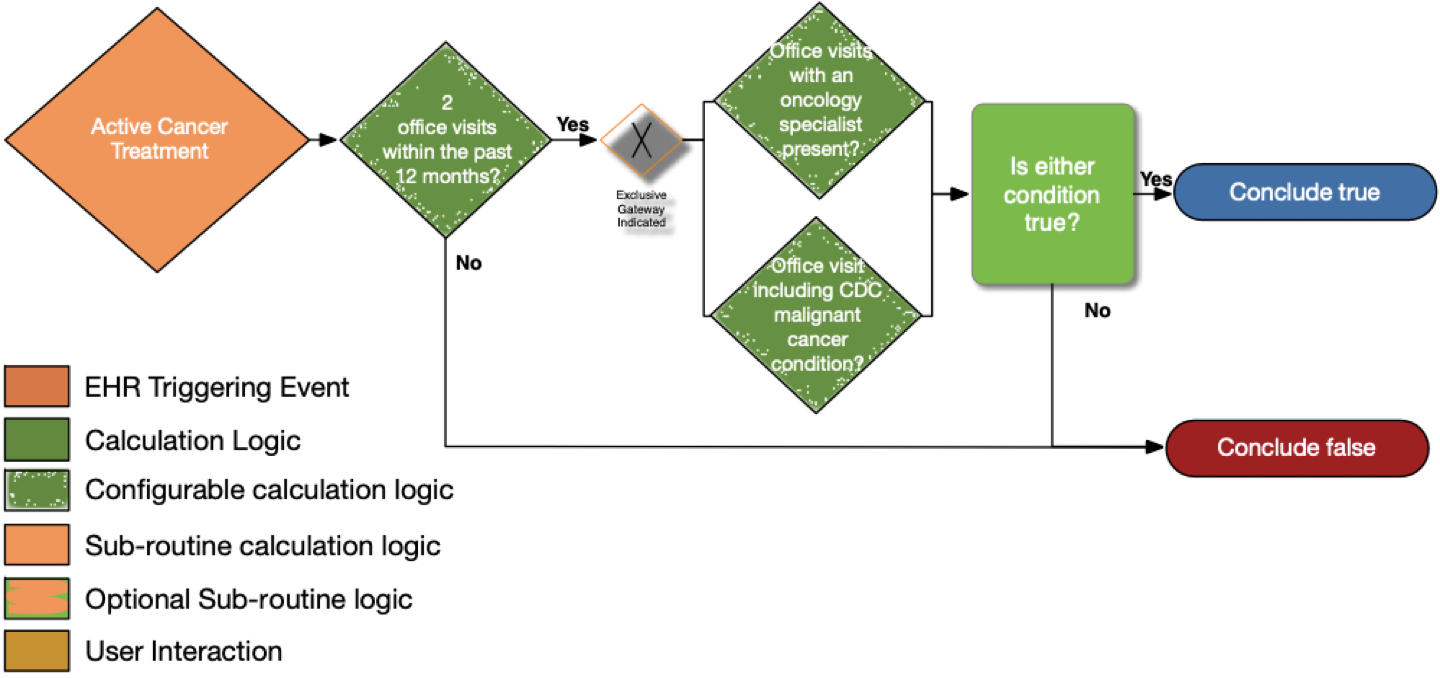
| Definition | Answer to Proceed | Details | Data (Terminology) Requirement | Profile | Path |
|---|---|---|---|---|---|
| Two office visits within the past 12 months? | No | Look for a minimum of two distinct encounters within 12 months of the date of the current visit for which each of the following is true: • the encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set |
Office Visit | Encounter | Encounter.class and Encounter.period.start |
| Office visits with an oncology specialist present? | No | • The encounter is performed by an oncologist as defined in the oncology specialty designations using the National Uniform Claim Committee (NUCC) classifications |
Oncology specialty designations (NUCC) | PractitionerRole and Encounter | PractitionerRole.specialty, Encounter.participant.type, and Encounter.participant.individual |
| Office visits including CDC malignant cancer condition? | No | • The encounter diagnosis (primary or secondary or co-morbidity diagnosis) is listed in the CDC Malignant Cancer Conditions value set | CDC malignant cancer conditions | Condition and Encounter | Condition.category, Condition.code, and Encounter.diagnosis |
The following artifacts formalize the description of the logic and behavior defined by this recommendation.
| Resource | Type | Description |
|---|---|---|
| 2022 CDC Clinical Practice Guideline Recommendation #7 | PlanDefinition | Event-Condition-Action rule that implements behavior for 2022 CDC Clinical Practice Guideline Recommendation #7 |
| Recommendation #7 - benefits and harms of starting and/or continuing opioid therapy for subacute or chronic pain | Library | Defines the data requirements to support evaluation of recommendation #7 |
| Opioid Terminology Management Knowledge-base Data (OMTK) Library | Library | CQL Library that provides logic for implementation of opioid management functionality including Milligram Morphine Equivalents (MME). |
| Opioid Terminology Management Knowledge-base (OMTK) Library | Library | CQL Library that provides logic for implementation of opioid management functionality including Milligram Morphine Equivalents (MME). |
| Common Opioid Decision Support Logic | Library | CQL Library that provides common logic for the recommendations |
| Common OpioidCDS Configuration Logic | Library | CQL Library that provides common configuration logic for the recommendations |
| Common OpioidCDS Routines Logic | Library | CQL Library that provides common routines logic for the recommendations |
| FHIRHelpers Conversion Logic | Library | CQL Library that defines functions to convert between FHIR data types and CQL system-defined types, as well as functions to support FHIRPath implementation |
| Description | CDS Hooks Request | Expected Response |
|---|---|---|
| Patient is 18 or older. Patient has been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502).one tablet per day for 30 days. Patient is also being prescribed maCarbinoxamineleate 0.4 MG/ML / Hydrocodone Bitartrate 1 MG/ML / Pseudoephedrine Hydrochloride 6 MG/ML Oral Solution (RXNorm 1012727) 3 tablets per day for 30 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502) 1 tablet per day for 30 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502) one tablet per 1 day for 7 days. Patient has also been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm one tablet per 1 day for 7 days. "Assessment of risk for opioid use procedure." Patients on opioid therapy should be evaluated for benefits and harms within 1 to 4 weeks of starting opioid therapy and every 3 months or more subsequently. Assessment of risk for opioid abuse (procedure) | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet. (RXNorm 1049502) one tablet per 1 day for 6 days. Patient has also been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (Rxnorm 0149502) one tablet per 1 day for 6 days.The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502) one tablet per 1 day for 30 days. Patient has also been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502) one tablet per 1 day for 63 days. This will trigger the message "No evaluation for benefits and harms associated with opioid therapy has been performed for the patient in the past 3 months" Patients on opioid therapy should be evaluated for benefits and harms within 1 to 4 weeks of starting opioid therapy and every 3 months or more subsequently. | Request JSON | Response JSON |
| Patient is 18 or older. Patient has been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502) 1 tablet per 1 day for 30 days. Patient has also been prescribed 12 HR Oxycodone Hydrochloride 10 MG Extended Release Oral Tablet (RXNorm 1049502) 1 tablet per 1 day for 62 days. The patient will be excluded and no message will be triggered - an empty set of cards will be returned. | Request JSON | Response JSON |
IG © 2019+ Centers for Disease Control and Prevention (CDC). Package fhir.cdc.opioid-cds-r4#2022.1.0 based on FHIR 4.0.1. Generated 2024-04-22
Links: Table of
Contents |
License |
QA Report
| New Issue | Issues
| Version History |