HIV Screening Clinical Guidelines Implementation Guide
0.1.0 - CI Build
Unknown region code '840'
HIV Screening Clinical Guidelines Implementation Guide
0.1.0 - CI Build
Unknown region code '840'
HIV Screening Clinical Guidelines Implementation Guide, published by National Association of Community Health Centers, Inc. (NACHC). This guide is not an authorized publication; it is the continuous build for version 0.1.0 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/cqframework/hiv-cds/ and changes regularly. See the Directory of published versions
| Official URL: http://fhir.org/guides/nachc/hiv-cds/ImplementationGuide/fhir.nachc.hiv-cds | Version: 0.1.0 | |||
| Draft as of 2024-03-22 | Computable Name: HIV_NACHC | |||
Copyright/Legal: Copyright National Association of Community Health Centers, Inc. (NACHC) |
||||
The purpose of this Implementation Guide is to provide a basis of Clinical Decision Support regarding the recommendation for the screening of HIV.
This project was supported by Centers for Disease Control and Prevention under Cooperative Agreement number NU38OT000310. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by CDC, HRSA, HHS, IP or the U.S. Government.
Implementing an evidence-based clinical decision support quality improvement (QI) initiative to improve health centers in HIV prevention, diagnoses, care, and treatment to improve health outcomes. NACHC strategic approach will leverage existing informatics clinical decision support infrastructure both internally and externally to advance efforts of health centers to implement evidence-based strategies to end the HIV epidemic in their communities.
NACHC and its partners are applying Human Centered Design Framework tools and strategies to develop a clinical decision support intervention that is care team and patient centric through:
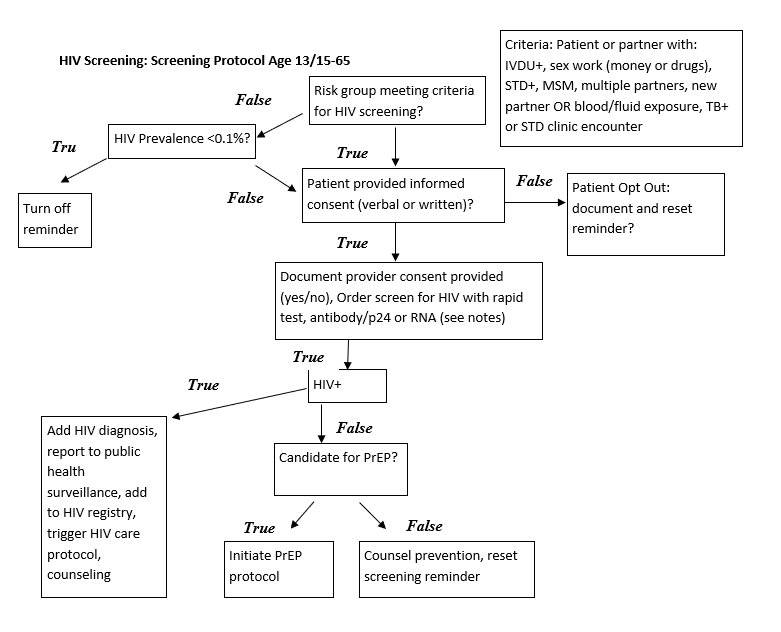
The main sections of this IG are:
RxNorm: This product uses publicly available data courtesy of the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product.
Nelson SJ, Zeng K, Kilbourne J, Powell T, Moore R. Normalized names for clinical drugs: RxNorm at 6 years.
J Am Med Inform Assoc. 2011 Jul-Aug;18(4)441-8. doi: 10.1136/amiajnl-2011-000116.
Epub 2011 Apr 21. PubMed PMID: 21515544; PubMed Central PMCID: PMC3128404.
Full text
UCUM: This product includes all or a portion of the UCUM table, UCUM codes, and UCUM definitions or is derived from it,
subject to a license from Regenstrief Institute, Inc. and The UCUM Organization. Your use of the UCUM table,
UCUM codes, UCUM definitions also is subject to this license, a copy of which is available at http://unitsofmeasure.org.
The current complete UCUM table, UCUM Specification are available for download at http://unitsofmeasure.org.
The UCUM table and UCUM codes are copyright © 1995-2009, Regenstrief Institute, Inc. and the Unified Codes for Units of Measures (UCUM) Organization.
All rights reserved.
THE UCUM TABLE (IN ALL FORMATS), UCUM DEFINITIONS, AND SPECIFICATION ARE PROVIDED "AS IS." ANY EXPRESS OR IMPLIED WARRANTIES ARE DISCLAIMED, INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE.
Bransom BM, et al. Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Wemon in Health-Care Settings — United States, 2006. MMWR Recmm Rep 2006;55(No. RR-14):1–17. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm.
DiNenno EA, Prejean J, Irwin K, et al. Recommendations for HIV Screening of Gay, Bisexual, and Other Men Who Have Sex with Men — United States, 2017. MMWR Morb Mortal Wkly Rep 2017;66:830–832. DOI: http://dx.doi.org/10.15585/mmwr.mm6631a3.
Skinner HA (1982). The Drug Abuse Screening Test. Addict Behav 7(4):363-371. Yudko E, Lozhkina O, Fouts A (2007). A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treatment 32:189-198.
National Institute on Drug Abuse (NIDA). Drug Abuse Screening Test (DAST-10). https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69. Accessed 2021-11-09.
Office of the National Coordinator for Health Information Technology (ONC), USCDI V2 Data Element: Sexual Orientation. https://www.healthit.gov/isa/uscdi-data/sexual-orientation. Accessed 2021-11-09.
Office of the National Coordinator for Health Information Technology (ONC), USCDI V2 Data Element: Gender Identity. https://www.healthit.gov/isa/uscdi-data/gender-identity. Accessed 2021-11-09.