Pharmaceutical Quality Submissions to Food & Drug Administration (PQ/CMC), published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 0.1.20 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/FHIR-us-pq-cmc/ and changes regularly. See the Directory of published versions
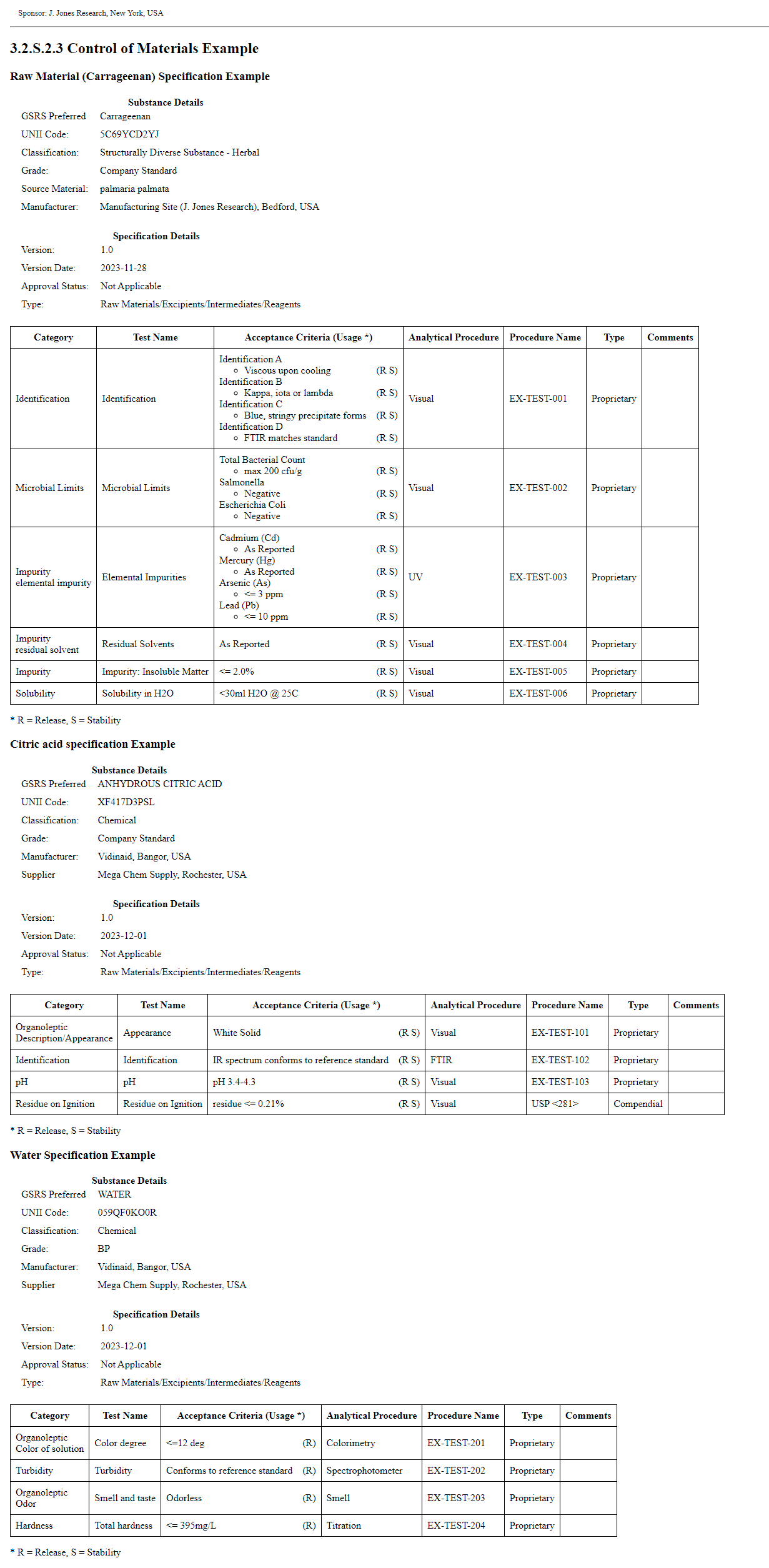
The Substance Control of Materials bundle profile provides a mechanism for the industry to submit Module 3 CTD 3.2.S.2.3 folder content to the FDA. This section provides evidence supporting the identity, composition, and origin of the raw materials used in the manufacture of the active substance.
In addition to identifying the raw material by name (e.g., GSRS Preferred, Generic, Common) and UNII, the content includes information such as: quality standard, identification of the source organism for biologically sourced materials, manufacturer, supplier, and as appropriate, evidence demonstrating that the materials meet the standards appropriate for their intended use.
The domain concepts of Substance Control of Materials are represented in FHIR in this IG. Below is a high-level FHIR resource mapping to guide the understanding of how the domain concepts are represented using profiles on FHIR Resources. Detail study of the profiles and each of the resources will be needed for developing a deeper understanding of this Substance Control of Materials FHIR Bundle Profile. Concepts that are key to this domain include the following:
Note: profile computable names (in parenthesis above) map to names in the Profile Map below.
See Usage Patterns on Quality Specification Profile
This example demonstrates a substance with three raw materials. One raw material has a source information backbone element instance. This image displays the narrative as inserted in the composition text element generated by the narrative transform. The XML can be found on the Artifacts page. The XML file with the publisher narrative is on the artifacts page and in the Bundle profile. 7ce2088d-d281-4e2f-9a25-120a1f9805d1