Pharmaceutical Quality Submissions to Food & Drug Administration (PQ/CMC), published by HL7 International / Biomedical Research and Regulation. This guide is not an authorized publication; it is the continuous build for version 0.1.20 built by the FHIR (HL7® FHIR® Standard) CI Build. This version is based on the current content of https://github.com/HL7/FHIR-us-pq-cmc/ and changes regularly. See the Directory of published versions
The Description and Composition of the Drug Product bundle profile provides a mechanism for the industry to submit Module 3 of CTD 3.2.P.1 folder content to the FDA. The contents of this section include a description of the drug product, its container closure, and its components and constituents. At this time, the profile is scoped to support only products that have a solid oral dose form; other dose forms will be supported in future iterations of the profile.
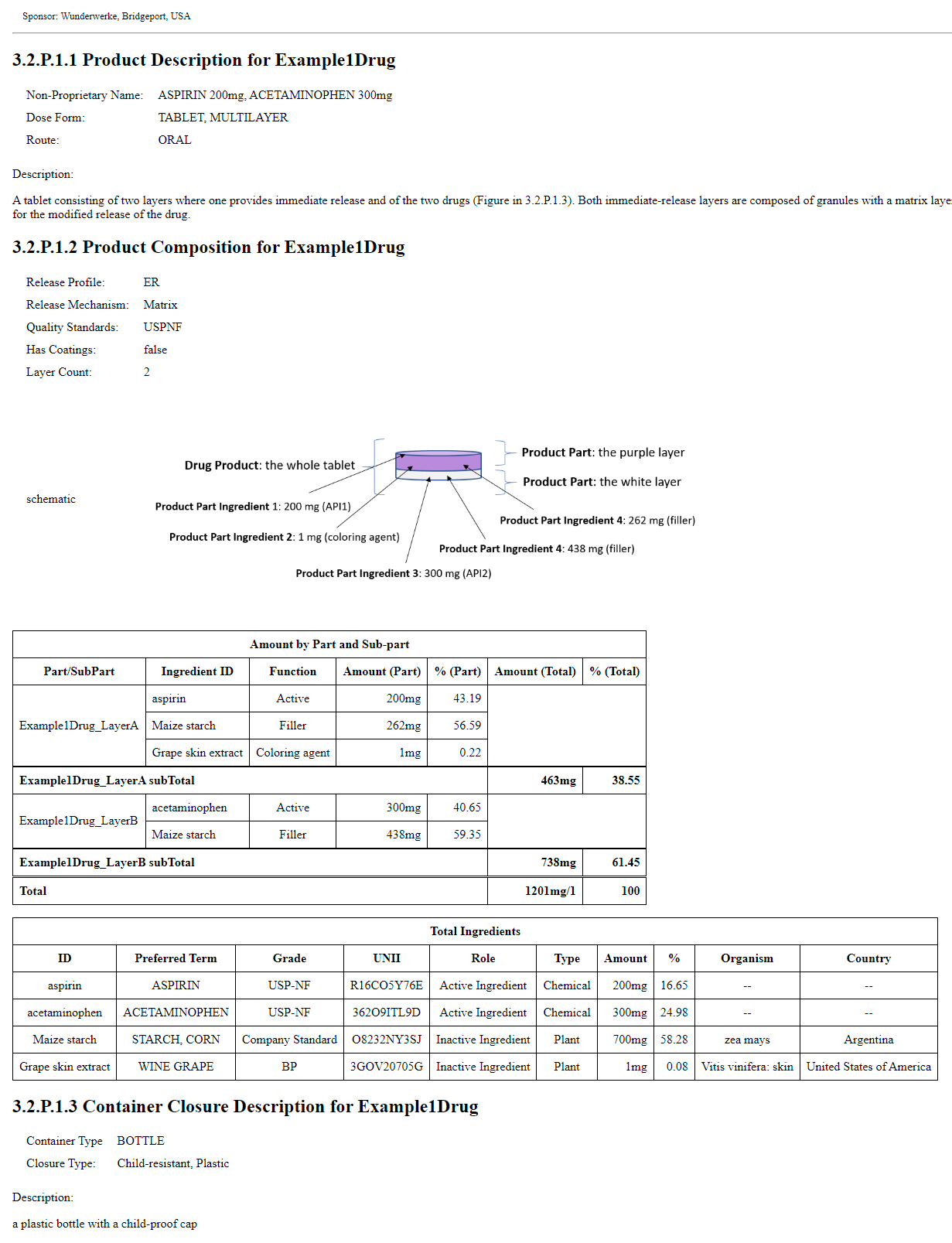
For the purposes of the PQ/CMC IG, the Example 1 below of a two-layer Tablet provides description of the terms used to explain the 2-layer tablet.
 |
Example 1: A 2 layer tablet with 1 purple layer and 1 white layer
The additional example illustrations below of drug products are included as an aid to understanding the terms or semantics in the context of the PQ/CMC IG.
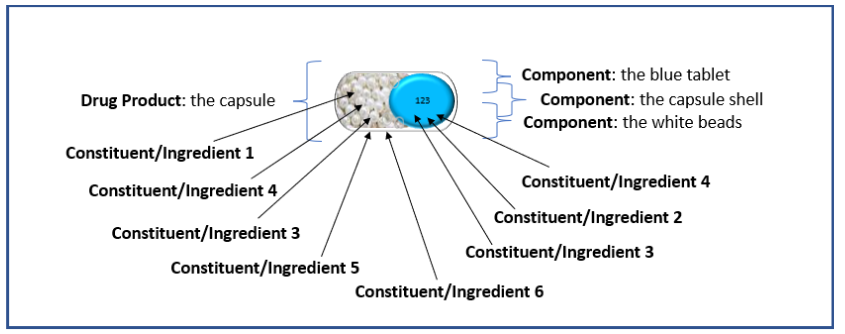 |
Example 2: Capsule with 3 components: capsule shell, one type of beads, and a minitablet
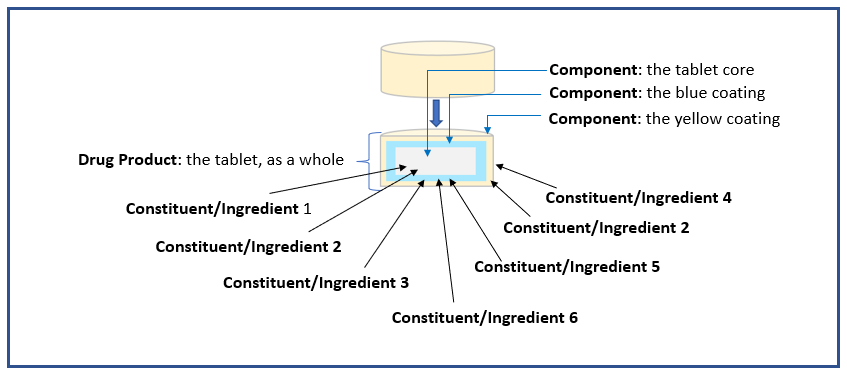 |
Example 3: Tablet with two coatings
Drug Product content includes information such as: the dosage form, routes of administration, a narrative description of the product, a schematic, the type of container and container closure used for the dosage form, quality standard and release profile of the dose unit, and the amount of each constituent (both active and inactive ingredients) contained in the drug product.
Component content includes information such as: a component identifier, component type, and release profile of the component and the amount of each constituent (both active and inactive ingredients) contained in the component as well as the function of the constituent in the component.
The domain concepts of Description and Composition of the Drug Product are represented in FHIR in this IG section. Below is a high-level FHIR resource mapping to guide the understanding of how the domain concepts are represented using profiles on FHIR resources. Detail study of the profiles and each of the resources will be needed to develop a deeper understanding of this Description and Composition of the Drug Product FHIR bundle profile. Concepts that are key to this domain include the following:
Note: profile computable names (in parenthesis above) map to names in the Profile Map below.
Not Applicable
This image demonstrates a multilayer tablet displayed with narrative inserted in the composition text element. It has two parts. The XML can be found on the Artifacts page and does not contain the narrative in the image, rather it contains the narrative generated for all examples by the IG publisher program. It is on the artifacts page and in the Bundle profile. bd0f0a7a-27ea-4884-801d-bd0546e80888